View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Common Mullein - Verbascum thapsus
Other Names:
Great Mullein, Velvet Plant, Woolly Mullein
Non-native Species
Global Rank:
GNR
State Rank:
SNA
(see State Rank Reason below)
C-value:
0
Agency Status
USFWS:
USFS:
BLM:
External Links
State Rank Reason (see State Rank above)
Verbascum thapsus is native to portions of Eurasia and was brought to North America as a medicinal herb beginning in the 18th Century, and through accidental introductions (Gross and Werner 1978). A conservation status rank is not applicable (SNA) because Verbascum thapsus is a non-native vascular plant in Montana that is not a suitable target for conservation activities.
General Description
PLANTS: Course, biennial (occasionally annual) forbs that grow erect from a simple or branched stem, 1.3 to 5 (10) feet tall. Plants are copiously tomentose with stellate hairs. Sources: Giblin et al. [eds.] 2018; Lesica et al. 2022
LEAVES: Basal leaves are simple with petioles and form a rosette; blades are oblanceolate to obovate, 5-40 cm long, with entire to shallowly serrate margins, and covered with soft, fine hairs. Cauline leaves are simple, sessile (lack petiole) and strongly decurrent on the stem, fuzzy (tomentose with branched hairs), alternately arranged, and reduced upwards on the stem. Stem leaf blades are lanceolate in shape. Sources: Giblin et al. [eds.] 2018; Lesica et al. 2022
INFLORESCENCE: A stout terminal, spike-like panicle with dense, sulfur-yellow flowers. The flower arrangement resembles a ‘corncob’. Flowers are radially symmetrical and sessile, in clusters of 2-10 within the axils of a bract. Sepals: 5, lanceolate, deeply divided but fused at base, and 4-10 mm long. Petals 5, fused,15-20 mm across. Stamens: 5, exserted. Filaments are slender with white to yellow long, soft, crooked, and unmatted hairs (villous). Anthers are yellow. Pistil: single with a capitate stigma. Sources: Giblin et al. [eds.] 2018; Lesica et al. 2022
TAXONOMY & NOMENCLATURE
Common Mullein has numerous common names, while its scientific classification has been stable since Carolus Linnaeus (1707-1778) published the Species Plantarum (1753). Consult the article, "Common Mullein-The Roadside Torch Parade” for an interesting read on this plant’s many common names and ancient uses (Mitich 1989).
Montana’s plants belong to subspecies thapsus (Giblin et al. [eds.] 2018). The specific epithet thapsus may have come from the Sicilian Isle of Thapsos, where in ancient times plants were gathered in abundance, or from the Tunisian island of Thapsus (Mitich 1989).
Pliny (Gaius Plinius Secundus), a Roman author, naturalist and natural philosopher, used the term “Verbascum” for “mullein” (Cochran 1948). The word ‘mullein’ comes from the Latin mollis which means ‘soft’ (Mitich 1989). It could have indirectly reached its present meaning as a derivative of the old English ‘muleyn’ which means ‘woolen’ (Mitich 1989).
Phenology
Flowering from June through September (Gross and Werner).
Diagnostic Characteristics
Montana’s two
Verbascum species can be distinguished by these characteristics:
Common Mullein -
Verbascum thapsus, non-native, aggressive
* Plants: Densely hairy (tomentose). Lacks glands.
* Leaves: Gray-green. Basal leaves grow more upwards/outwards. Stem leaves velvety or wooly (tomentose) and long-decurrent.
* Inflorescence: Not glandular.
* Flowers: Sessile in little clusters.
Moth Mullein -
Verbascum blattaria, non-native
* Plants: Glabrous or puberulent; glandular.
* Leaves: Brighter green. Basal leaves flattened to ground. Lower stem leaves glabrous.
* Inflorescence: Glandular-hairy.
* Flowers: Pedicels, 5-15 mm long.
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Common Mullein is native to Eurasia (Lesica et al. 2022). Plants were introduced into North American several times as a medicinal herb and accidentally, and predominantly from Europe (Gross and Werner 1978). Common Mullein was imported to the Blue Ridge Mountains of Virginia in the mid-1700s. By 1818 plants were reported as widespread. By 1839 it was present in the Midwest to Michigan (Eaton 1818), and by 1880 was in California (Watson 1880).
In Montana, the earliest documented herbarium specimen of Common Mullein was found in 1892 in Meagher County (CPNWH 2025; CGPH 2025). In 1895, plants were documented in Gallatin County and by 1900 were recorded from Flathead County (CPNWH 2025; CGPH 2025). As of 2024, Common Mullein has been documented in nearly every county of Montana.
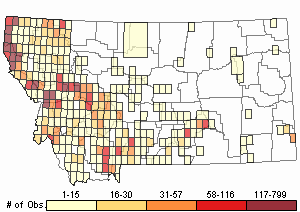
Observations in Montana Natural Heritage Program Database
Number of Observations: 11273
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
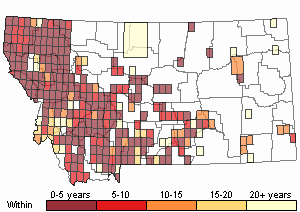
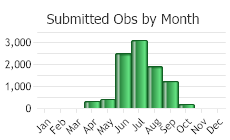
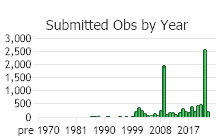
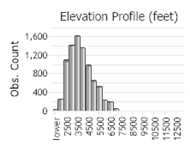 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Common Mullen grows on disturbed, often stony soil of grasslands, meadows, open forest, talus slopes, roadsides in the plains and valley zones of Montana (Lesica et al. 2022). In addition, plants are found in rangeland, forest clearings, and meadows (Ditomaso et al. 2013). Plants prefer disturbed sites with well-drained soils; however, they are not limited to these conditions (Ditomaso et al. 2013).
Ecology
GROWTHCommon Mullein is known as an Earth-regenerator because populations are early colonizers of barren or highly disturbed soils - and typically are ephemeral (Gross and Werner 1978; Wulff-Tilford 1993). In Yellowstone National Park, the distribution of exotic plant species was studied relative to vehicle-accessible campgrounds (Allen and Hansen 1999). Common mullein was found where overstory canopy cover was less than 30%, and at three-quarters of the occurrences was 5% or less.
Plants are cold hardy and drought resistance (Wulff-Tilford 1993). Studies examining the growth and development of Common Mullein have been summarized by Gross and Werner (1978). Common Mullein photosynthesizes at similar rates over a wide spectrum of cold to warm temperatures, which was determined to aid its ability to colonize a diversity of habitats (Gross and Werner 1978). The plant’s fuzzy cover sourced from dense, branched hairs has been the subject of many research projects. Experiments comparing shaved and unshaved leaves studied the effects of the hairs on transpiration. Researchers concluded that the dense hairs primarily increased stomatal resistance and only slightly increased the leaf’s boundary layer resistance. The dense hairs also prevent ice crystals from forming on the leaf’s underlying tissues. Low temperatures trigger degradation of starch roots which is presumed to protect the over-winter rosettes. The species is not known to be allelopathic.
CULTURAL & MEDICINAL USESCommon Mullein has a long history of being used for medicines. American Mullein products are derived from
Verbascum thapsus while European Mullein products are from
Verbascum densiflorum (
Drugs.com). Preparations of the plant have been ingested, applied topically, and smoked (
Drugs.com). The flowers, inflorescence, leaves, and potentially the roots are the typical parts used by herbalists (Wulff-Tilford 1993). Common Mullein is used as an expectorant, vulnerary, demulcent, diuretic, and in numerous other ways (Wulff-Tilford 1993). As of 2024 no pharmaceutical drugs have been manufactured from its components (
Drugs.com). Plants are used as a cough remedy and in the treatment of earaches (Wulff-Tilford 1993). The root is supposedly useful for treating bladder incontinence (Wulff-Tilford 1993). The yellow flowers have been used to produce a hair dye (
Drugs.com). Common Mullein is not known to be allelopathic, allergenic, or poisonous to humans, though studies on toxicity are limited (
Drugs.com; Gross and Wern 1978).
Common Mullein has other cultural uses. Native Americans and Early Americans used the leaves to line their shoes.
PLANT-INVERTEBRATE ANIMAL INTERACTIONSFrom 2017 to 2022 a study of the insect fauna at a stream restoration project was conducted along Sevenmile Creek near Helena, Montana (Sater 2022). Common Mullein is known to support or be a host plant for the following insect species, which were also directly observed at the restoration site:
*
Brown Stink bug - Euschistus servus. In spring, Common Mullein is thought to provide an early source of food.
* Larval stages of moths in the genus
Caradrina * Larval stages of butterflies in the genus
Euphydryas.
*
Cosmopepla lintnerianaPOLLINATORSCommon Mullein is visited by a diversity of insects, as summarized by Gross and Werner (1980). Short- and long-tongued bees are the more effective pollinators for cross-pollination. Hover-flies also pollinate flowers. Flowers can also self-pollinate.
The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus vagans,
Bombus ternarius, and
Bombus pensylvanicus (Colla and Dumesh 2010).
Reproductive Characteristics
Plants reproduce by seed.
FLOWERING [adapted from Gross and Werner (1978)]
Each flower is ephemeral, opening before dawn and closing in mid-afternoon on the same day. The pistil’s style matures first and bends downward once the anthers have matured. The two naked, anterior stamens produce most of the fertilizing-pollen. It is presumed that the three posterior stamens provide a landing spot for pollinators. As illustrated by Gross and Werner (1978), hover-flies gather nectar and pollen from the plant's posterior anthers while the anterior anthers deposit pollen on the insect's abdomen. If not cross-pollinated by the end of the day then flowers will self-pollinate. As the corolla closes, the style returns to its original position which pushes the receptive stigma against the anthers to self-pollinate.
FRUITS
Fruit is a capsule. Capsule is broadly ellipsoid, 7-10 mm long, and 2-celled. At maturity, capsules open length-wise to disperse numerous seeds (Giblin et al. [eds.] 2018). A study by Salisbury (1942) estimated an average of 226 ± 42 capsules per plant and an average of 596 ± 30 seeds per capsule, which averages to about 136,000 seeds per plant (in Gross and Werner 1978). Another study in 1976 calculated 175,000 seeds per plant (Gross and Werner 1978).
Seeds are gray and taper similar to a corn cob (Meehan 1884).
LIFE CYCLE [adapted from Gross and Werner (1978) and DiTomoso et al. (2013)]
Common Mullein populations are ephemeral and require periodic disturbances to sustain populations. Otherwise, other plant species growing densely in an undisturbed setting can displace Common Mullein. Seedlings germinate in early spring, and/or after a disturbance event. Seedlings develop a rosette that grows throughout the summer and into late fall. Establishment is greatly enhanced where the ground is barren. Upon being subjected to lower temperatures (winter), the plant produces a tall flower stalk of indeterminate growth. Flowering occurs in late June and peaks in August. On the stalk, flowers mature from the bottom to the top in successive spirals. As the season progresses, each successive spiral overlaps for most of its length with the former spirals. Upon maturity, movement by the wind or an animal causes the capsule to longitudinally split, releasing seeds. A 1956 study found that 75% of seeds fell within 1 meter, 18% within 5 meters, and 7% within 11 meters of the parent plant. A mature plant can produce more than 100,000 seeds. Seeds in the soil can survive for more than 35 years. Viable seeds were found from an archeological site in Denmark that dated to 1300 A.D.
Because it is a biennial, roots reach their peak development in the 1st year and tend to dry after flowering in the 2nd year.
Management
COUNTY & STATE DESIGNATIONSAs of 2024, Common Mullein is listed as
County Noxious in 12 counties of Montana.
In 1999, 2006, and 2013, Common Mullein was petitioned for State-listing as noxious. The Montana Department of Agriculture manages criteria for listing, and records pertaining to petitioned non-native plant species. To date, the evaluating committees determined - in part - that listing as State Noxious was not warranted because plants can be effectively controlled in cropland, are early colonizers, and establish where the soil and ground have been disturbed.
INTEGRATED VEGETATION MANAGEMENTAn integrated vegetative management approach provides the best long-term control, and requires that land-use objectives and a desired plant community be identified (Shelly et al. in Sheley and Petroff 1999). Once identified, an integrated weed management strategy that promotes a weed-resistant plant community and serves other land-use objectives such as livestock forage, wildlife habitat, or recreation can be developed.
PREVENTION [Adapted from Piper in Sheley and Petroff 1999]
Preventing the establishment of Common Mullein is the most effective control method and fairly easy to accomplish by many practices:
* Learn how to accurately identify Common Mullein in order to detect occurrences early and minimize invasions.
* Prevent vehicles from driving through, and animals from grazing within, infested areas.
* Thoroughly wash the undercarriage of vehicles and wheels in a designated area before moving to an un-infested area.
* Frequently monitor for new plants, and when found implement effective control methods.
* Maintain proper grazing management that creates resilience to noxious weed invasion.
* Plant or seed native species in barren areas, especially where sites are naturally and historically vegetated.
PHYSICAL and CULTURAL CONTROLS [Adapted from Harvey and Mangold 2018]
Hand-pulling plants before seed-set is effective, especially if growing on loose soil. When digging, sever the root below the soil surface. Soil disturbance stimulates recruitment. Hand-pulling is the most effective control method. Even if in the early phases of invasion, repeated hand-pulling can effectively remove plants over a couple of years. since plants only live
Mowing repeatedly during the bolting to early flowering stages can reduce seed production. Plants produce rosettes that cannot be effectively mowed and quickly regrow if mowing is discontinued.
Disking/Tillage is effective for controlling existing plants, but soil disturbance stimulates recruitment. Common Mullein is not competitive in places where there is annual cropping.
Fire is not an effective control. Fire stimulates, often dramatically, germination and establishment. Fire can be used to manage the seedbank, if seedlings are controlled with fire.
Revegetating with native or desirable plants is an effective control method. Populations are ephemeral. Therefore, with time populations usually decline unless periodically disturbed. Promoting competitive native or desirable plant species and reducing soil disturbance are the best control options. Native vegetation that shades and competes for early season moisture will be better at reducing Common Mullein germination.
BIOLOGICAL CONTROLS Mullein Weevil (
Rhinusa tetra) specifically attacks both Common and Moth Mullein (Winston et al. 2014). The larvae mature within the seed capsules and destroy up to 50% of the seeds (DiTomaso et al. 2013) Mullein Weevil was accidentally introduced from Europe into the USA in 1919 from an unknown source (Winston et al. 2014). In 1995 it was deliberately re-distributed (Winston et al. 2014). As of 2014, Mullein Weevil is considered to be well-established in Washington, where it has been shown to cause extensive seed destruction. In Oregon it is widespread, but the impact is unknown. Mullein Weevil occurs in California, but has had little impact. In Montana, populations are limited and impacts are unknown.
Mullein Leaf Bug (
Campylomna verbasci) normally feeds on mullein plants. In Nova Scotia, Canada the Mullein Leaf Bug has caused extensive damage to apple and pear trees and potato leaves (Maw 1980; Pickett 1939). In British Columbia, Mullein Leaf Bug was shown to cause little economic damage (Madsen et al. 1975). Mullein Leaf Bug may also be beneficial because it must also feed on several mite and insect species, known to affect fruit trees, in order to complete its life cycle.
Mullein Moth <
Cucullia verbasci) was tested as a bio-control agent for
Verbascum thapsus (Maw 1980). The study used Mullein Moth pupae obtained from Europe and Maw concluded that Mullein Moth is a safe agent to release against mullein. Further information has not been found and interested readers should consult Maw 1980.
CHEMICAL CONTROLS [Adapted from Harvey and Mangold 2018]
Herbicides with a surfactant can be effective, especially when properly managed with other tactics. A surfactant is necessary because the dense, branched hairs prevent the solutions of 2,4-D (for example) from reaching the leaf’s tissue. The herbicide type and concentration, timing of chemical control, soil properties, and other factors will determine its effectiveness and impact to non-target species. Strict adherence to application requirements defined on the herbicide label will reduce risks to human and environmental health. Many herbicides must be applied by applicators with an Aquatic Pest Control license. Consult your County Extension Agent and/or Weed District for more information on herbicidal control.
Common Mullein completes its life cycle in two years (biennial), producing leaves in the 1st year and flowering stems that fruit in the 2nd year.
Often landowners apply herbicides after the plant has bolted which is too late; this causes the stem to bend downward (epinasty curving) before eventually re-righting itself with continued growth.
Herbicides should only be applied during the 1st year of growth when plants are in seedling, rosette, or very early bolting stages. DiTomaso et al. (2013) has summarized
chemical control for mullein species.
GRAZING CONTROLS [Adapted from DiTomasa et al 2013)]
At all life stages, Common Mullein is not palatable for domesticated livestock and native ungulates, such as deer, elk, pronghorn, and bighorn sheep. Livestock usually avoid the plant because the thick leaves are densely hairy.
When present in rangelands, Common Mullein is an indicator that the site is over-grazed.
PARASITESSix species of leaf-inhabiting parasitic fungi have been reported on Common Mullein (Gross and Werner 1978).
Useful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuides
Stewardship Responsibility
Threats or Limiting Factors
Common Mullein is an aggressive, non-native plant. In rangelands it invades over-grazed, sparsely vegetated, and barren land while providing no nutritional forage for livestock or native ungulates (Gross and Werner 1978). Plants also invade sparsely vegetated soils in moist meadows and drainages. Plants can outcompete and displace native plant species where vegetative cover is low because it grows fast and is a prolific seeder. Specifically, after fire, Common Mullein can rapidly establish. Although it has medicinal properties, if left to seed Common Mullein will become invasive in your garden or yard with seeds; seeds will remain viable for a very long time.
Common Mullein is not a serious agricultural weed because annual tilling of the soil controls their populations (Gross and Werner 1978). However, plants can harbor other organisms that are destructive to commercially important plants. Powdery Mildew (Erysiphe cichoracearum) can affect vegetables and garden plants such as Cucumis, Helianthus, and Dahlia. Root Rot (Phymatotrichum omnivorum) is a pathogen that affects cotton plants. It’s important to know that a pathogen reported on a wild plant is not necessarily of the same strain that would affect a crop plant and may not be transferable.
Due to its prolific seed crop and velvety leaves, Common Mullein can be difficult to eradicate. This is especially true if not detected and controlled early.
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Allen, Karen, and Katherine Hansen. 1999. Geography of Exotic Plants Adjacent to Campgrounds, Yellowstone National Park, USA. Great Basin Naturalist 59(4), pp. 315-322.
Allen, Karen, and Katherine Hansen. 1999. Geography of Exotic Plants Adjacent to Campgrounds, Yellowstone National Park, USA. Great Basin Naturalist 59(4), pp. 315-322. Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68. DiTomaso, Joe M., and G.B. Kyser. 2013. A Weed Report from Weed Control in Natural Areas in the Western United States. Weed Research and Information Center, University of California. 544 pp.
DiTomaso, Joe M., and G.B. Kyser. 2013. A Weed Report from Weed Control in Natural Areas in the Western United States. Weed Research and Information Center, University of California. 544 pp. Gross, Katherine L., and Patricia A. Werner. 1978. The Biology of Canadian Weeds. 28. Verbascum thapsus L. and V. blattaria L. Can. J. Plant Sci. 58: 401-413
Gross, Katherine L., and Patricia A. Werner. 1978. The Biology of Canadian Weeds. 28. Verbascum thapsus L. and V. blattaria L. Can. J. Plant Sci. 58: 401-413 Hitchcock, C.L. and A. Cronquist. 2018. Flora of the Pacific Northwest: An Illustrated Manual. Second Edition. Giblin, D.E., B.S. Legler, P.F. Zika, and R.G. Olmstead (eds). Seattle, WA: University of Washington Press in Association with Burke Museum of Natural History and Culture. 882 p.
Hitchcock, C.L. and A. Cronquist. 2018. Flora of the Pacific Northwest: An Illustrated Manual. Second Edition. Giblin, D.E., B.S. Legler, P.F. Zika, and R.G. Olmstead (eds). Seattle, WA: University of Washington Press in Association with Burke Museum of Natural History and Culture. 882 p. Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Maw, M.G. 1980. Cucullia verbasci an Agent for the Biological Control of Common Mullein (Verbascum thapsus). Weed Science, Volume 28, Number 1, January, pp. 27-30.
Maw, M.G. 1980. Cucullia verbasci an Agent for the Biological Control of Common Mullein (Verbascum thapsus). Weed Science, Volume 28, Number 1, January, pp. 27-30. Meehan, Thomas. Immediate Influence of Crossing or Hybridizing on Fruits and Seeds. Bulletin of the Torrey Botanical Club. Volume 11, Number 10, page 119.
Meehan, Thomas. Immediate Influence of Crossing or Hybridizing on Fruits and Seeds. Bulletin of the Torrey Botanical Club. Volume 11, Number 10, page 119. Mitich, Larry W. Common Mullein-The Roadside Torch Parade. Weed Technology. Volume 3:704-705.
Mitich, Larry W. Common Mullein-The Roadside Torch Parade. Weed Technology. Volume 3:704-705. Sater, S. 2022. The insects of Sevenmile Creek, a pictorial guide to their diversity and ecology. Undergraduate Thesis. Helena, MT: Carroll College. 242 p.
Sater, S. 2022. The insects of Sevenmile Creek, a pictorial guide to their diversity and ecology. Undergraduate Thesis. Helena, MT: Carroll College. 242 p. Telewski, Frank W., and Jan A.D. Zeevaart. 2002. The 120-year period for Dr. Beal's Seed Viability Experiment. American Journal of Botany 89(8): 1285-1288.
Telewski, Frank W., and Jan A.D. Zeevaart. 2002. The 120-year period for Dr. Beal's Seed Viability Experiment. American Journal of Botany 89(8): 1285-1288. Toole, Vivian K. 1986. Ancient Seeds: Seed Longevity. Journal of Seed Technology. Volume 10, Number 1, pp. 1-23.
Toole, Vivian K. 1986. Ancient Seeds: Seed Longevity. Journal of Seed Technology. Volume 10, Number 1, pp. 1-23. Winston, R.L., M Schwarzlander, H.L. Hinz, M.D. Day, M.J.W. Cock, and M.H. Julien, Eds. 2014. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds, 5th edition. USDA Forest Service, Forest health Technology Enterprise team, Morgantown, West Virginia. FHTET-2014-04. 838 pp.
Winston, R.L., M Schwarzlander, H.L. Hinz, M.D. Day, M.J.W. Cock, and M.H. Julien, Eds. 2014. Biological Control of Weeds: A World Catalogue of Agents and Their Target Weeds, 5th edition. USDA Forest Service, Forest health Technology Enterprise team, Morgantown, West Virginia. FHTET-2014-04. 838 pp.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Ament, R.J. 1995. Pioneer Plant Communities Five Years After the 1988 Yellowstone Fires. M.Sc. Thesis. Bozeman, MT: Montana State University. 216 p.
Ament, R.J. 1995. Pioneer Plant Communities Five Years After the 1988 Yellowstone Fires. M.Sc. Thesis. Bozeman, MT: Montana State University. 216 p. Boggs, K. W. 1984. Succession in riparian communities of the lower Yellowstone River, Montana. M.S. Thesis. Montana State University, Bozeman, 107 pp.
Boggs, K. W. 1984. Succession in riparian communities of the lower Yellowstone River, Montana. M.S. Thesis. Montana State University, Bozeman, 107 pp. Cope, M.G. 1992. Distribution, habitat selection and survival of transplanted Columbian Sharp-tailed Grouse (Tympanuchus phasianellus columbianus) in the Tobacco Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 60 p.
Cope, M.G. 1992. Distribution, habitat selection and survival of transplanted Columbian Sharp-tailed Grouse (Tympanuchus phasianellus columbianus) in the Tobacco Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 60 p. Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp.
Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp. DuBois, K.L. 1979. An inventory of the avifauna in the Long Pines of Southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 113 p.
DuBois, K.L. 1979. An inventory of the avifauna in the Long Pines of Southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 113 p. Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p.
Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p. Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p.
Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p. Forcella, Frank, and Stephen J. Harvey. 1988. Patterns of Weed Migration in Northwestern U.S.A. Weed Science. Volume 36:-194-201.
Forcella, Frank, and Stephen J. Harvey. 1988. Patterns of Weed Migration in Northwestern U.S.A. Weed Science. Volume 36:-194-201. Gobeille, J.E. 1992. The effect of fire on Merriams turkey brood habitat in southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 61 p.
Gobeille, J.E. 1992. The effect of fire on Merriams turkey brood habitat in southeastern Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 61 p. Jensen, P.D. 2001. The foraging and nesting behavior of four solitary-nesting bee species (Hymenoptera: Megachilidae) in the Gallatin Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 76 p.
Jensen, P.D. 2001. The foraging and nesting behavior of four solitary-nesting bee species (Hymenoptera: Megachilidae) in the Gallatin Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 76 p. Jones, W. W. 1901. Preliminary flora of Gallatin County. M.S. Thesis. Bozeman, MT: Montana State College. 78 pp.
Jones, W. W. 1901. Preliminary flora of Gallatin County. M.S. Thesis. Bozeman, MT: Montana State College. 78 pp. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p.
Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p. Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p.
Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p. Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park.
Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park. Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p.
Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p. Rens, E.N. 2003. Geographical analysis of the distribution and spread of invasive plants in the Gardiner Basin, MT. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p.
Rens, E.N. 2003. Geographical analysis of the distribution and spread of invasive plants in the Gardiner Basin, MT. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p. Semmens, W.J. 1996. Seasonal movements and habitat use of the Highlands/Pioneer Mountains bighorn sheep herd of southwest Montana. M. Sc. Thesis. Bozeman, MT: Montana State University. 103 p.
Semmens, W.J. 1996. Seasonal movements and habitat use of the Highlands/Pioneer Mountains bighorn sheep herd of southwest Montana. M. Sc. Thesis. Bozeman, MT: Montana State University. 103 p. Simanonok, M. 2018. Plant-pollinator network assembly after wildfire. Ph.D. Dissertation. Bozeman, MT: Montana State University. 123 p.
Simanonok, M. 2018. Plant-pollinator network assembly after wildfire. Ph.D. Dissertation. Bozeman, MT: Montana State University. 123 p. Simanonok, M.P. and L.A. Burkle. 2019. Nesting success of wood-cavity-nesting bees declines with increasing time since wildfire. Ecology and Evolution 9:12436-12445.
Simanonok, M.P. and L.A. Burkle. 2019. Nesting success of wood-cavity-nesting bees declines with increasing time since wildfire. Ecology and Evolution 9:12436-12445. Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp.
Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp.
- Web Search Engines for Articles on "Common Mullein"





