View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Yellow Toadflax - Linaria vulgaris
Other Names:
Butter-and-eggs, Butter and Eggs
General Description
PLANTS: Taprooted perennial forbs that are somewhat woody at the base. Stems are clustered, erect, mostly simple (little branched), and 15–60 cm tall. Plants are most glabrous and pale green. Glandular hairs can be present in the upper stems/flowers. Sources: Lajeunesse in Sheley and Petroff 1999; Jacobs and Sing 2007; Lesica et al. 2012.
LEAVES: Alternate on the stem and numerous. Blades are narrow, linear-oblanceolate, and 1–5 cm long. Leaves are somewhat pointed on both ends.
INFLORESCENCE: A terminal bracteate raceme. Light yellow (butter-colored) flowers with orange centers and yellow spurs are snapdragon shaped and grow from the upper leaf nodes (leaf base).
The name “Toadflax” is in reference to its similarity to flax and is derived from “Tode Flax” or wild flax (Jacobs and Sing 2007). Carl Linnaeus derived the genus, Linaria, from Linum, the genus for Flax (Jacobs and Sing 2007). The specific epithet, vulgaris means “common”(Jacobs and Sing 2007).
Phenology
Flowering can begin in May and continues until October. Plants flowers along the upper stems while producing seed in the lower flowers. Source: (Lajeunesse in Sheley and Petroff 1999).
Diagnostic Characteristics
Montana’s two exotic Toadflax species are both rhizomatous with similar snapdragon type flowers, but are easily separated by their leaves. Hybrids between the species can be produced in the lab, but are not known to occur under natural conditions (Lajeunesse
in Sheley and Petroff 1999).
Linaria vulgaris leaves are linear, more than 8 times as along as wide, and do not clasp the stem.
Linaria dalmatica leaves are ovate, less than 8 times as long as wide, and clasp around the stem.
In the vegetative stage,
Leafy Spurge (Euphorbia virgata) resembles Yellow Toadflax. The leaves of Leafy Spurge are glabrous, linear-oblanceolate in shape, 2-6 cm long, and with entire margins.
Species Range
Montana Range
Range Descriptions
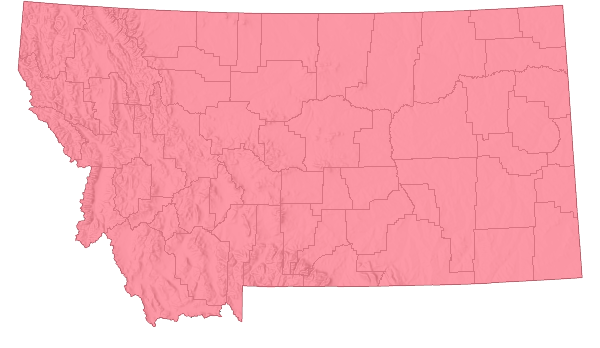
 Non-native
Non-native
Range Comments
Native to south-central Eurasia, where for centuries Yellow Toadflax was used as a medicine and fabric dye (Lajeunesse in Sheley and Petroff 1999) and as an insecticide in animal bedding (Jacobs and Sing 2007). In the late 1600s it was introduced into New England as a garden ornamental (Lajeunesse in Sheley and Petroff 1999). By the 1950s it had spread throughout North America and is most common in the northeastern U.S. and southeastern Canada, and more localized in western North America (Lajeunesse in Sheley and Petroff 1999).
It Montana it was first documented in Big Horn County in 1901 (www.pnwherbaria.org) though it had been reported in Flathead County in 1899 (Jacobs and Sing 2007).
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
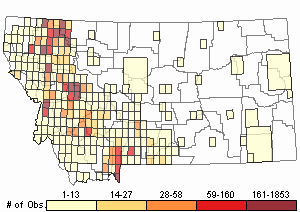
Observations in Montana Natural Heritage Program Database
Number of Observations: 10208
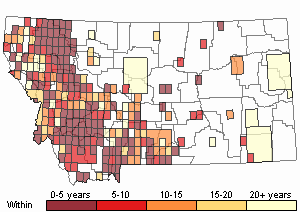
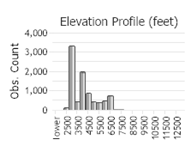
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
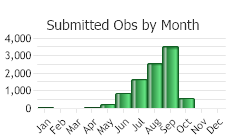
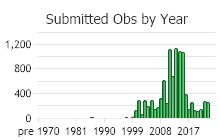
 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Roadsides, dry fields, grainfields, waste areas, gravel pits, pastures and rangeland, clearings, clearcuts, vacant lots, and railroad yards. In the plains, valleys, montane, and subalpine habitats in Montana. Sources: Lajeunesse in Sheley and Petroff 1999; Lesica et al. 2012.
In Montana and Idaho, localized infestations can grow at high elevations (MTNHP Botanist personal communication). In the Big Sky area of Montana logging operations from the 1960’s to 1980’s created localized infestations, which have been spreading from residential development.
Ecology
Yellow Toadflax has high genetic variability which allows it to occupy a wide range of conditions (Lajeunesse
in Sheley and Petroff 1999). They often grow in well-drained, coarse gravels to sandy loam soils, but can occur in heavier soils. Vulnerable habitats are areas where competition between species is low, sparsely vegetated soils, and drier, open areas on rangelands, particularly on south to southeast aspects. Populations can spread into adjacent more intact habitats. Yellow Toadflax can displace existing plant communities and desirable plants, cause loss of forage for domestic livestock and some big game, and reduce habitat for associated native animals. Toadflax species can provide cover for small mammals and native animals have been observed to browse on Dalmatian Toadflax or eat their seeds, but significant use has not been documented (Roboker 1970; Lajeunesse
in Sheley and Petroff 1999).
POLLINATORS The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus vagans,
Bombus appositus,
Bombus bifarius,
Bombus centralis,
Bombus fervidus,
Bombus flavifrons,
Bombus huntii,
Bombus melanopygus,
Bombus mixtus,
Bombus ternarius,
Bombus terricola,
Bombus occidentalis,
Bombus bimaculatus, and
Bombus griseocollis (Plath 1934, Macior 1974, Thorp et al. 1983, Colla and Dumesh 2010, Koch et al. 2012, Miller-Struttmann and Galen 2014, Williams et al. 2014).
Reproductive Characteristics
Plants reproduce from vegetative buds on deep-rooted rhizomes and by seeds (Lajeunesse in Sheley and Petroff 1999). New infestations of Dalmatian Toadflax often start from seed, but populations expand by both seed and vegetative reproduction. Vegetative shoots allow plants to grow earlier than other species and growth is often not limited by moisture.
FLOWERS
Calyx (sepals) is 3–5 mm long and green with lanceolate lobes. Corolla (petals) is yellow, 10–18 mm long, and with a straight spur measuring an additional 8–14 mm long. The corolla is bilabitate (split into an upper and lower corolla lip). The upper corolla is two-lobed. The lower corolla lip is 6–9 mm long, has 3 lobes that reflex down, and has an orange raised palette. There are 4 stamens and 1 stigma. The capsule is 6–8 mm long. Flowers begin growing in clusters, but spread apart on the stem as the season progresses. Source: Lajeunesse in Sheley and Petroff 1999; Lesica et al. 2012.
LIFE CYCLE [Adapted from Lajeunesse in Sheley and Petroff 1999]
Flower bud formation begins when Yellow Toadflax plants reach 16-24 inches tall. Yellow Toadflax capsules contain from 10-40 seeds, with each plant producing at estimated 15,000 to 30,000 seeds, which is far less than Dalmatian Toadflax. Seed dispersal begins in August or September and continues until winter in northern climates. Most seeds disperse during the growing season, but floral stalks can remain standing for two years and retain viable seeds. Seeds can be dispersed by wind as reported in several older publications. However, a study on Yellow Toadflax dispersal showed 80-90% of seeds fall within 18 inches of the parent plant. Snowmelt and rain help disperse seeds further.
Germination can be immediate or may require wet stratification (Jacobs and Sing 2007). Germination can occur in the fall, but predominately occurs the following April to May. Yellow Toadflax germination rates vary a lot, but are usually less than 10%. Most seeds that don’t germinate are dormant, and can be dormant for up to 10 years. Dormancy can be broken by cold followed by alternating warm and cool temperatures (Jacobs and Sing 2007). Seed viability has been shown to be 10-40%, but some seeds are just not viable. In Alberta, Canada Yellow Toadflax seedlings emerged in early to mid-May. A second, smaller flush of germination can occur in fall.
Due to their low seed production and viability, vegetative reproduction is more important for survival of a patch. Mature Yellow Toadflax roots can penetrate at least 3 feet deep. Lateral roots spread horizontally for several yards at depths from 2-8 inches.
Yellow Toadflax plants can live up to 4 years, but vegetative growth makes it difficult to age individuals.
Management
See
Noxious Weed Education VideoSuccessful control requires the use of several management strategies because of the plants genetic variability. Strategies for Yellow Toadflax should focus on preventing vegetative spread more than on reducing seed production. Once infestations are established, control will be labor-intensive, costly, and difficult.
PREVENTION [Adapted from Lajeunesse
in Sheley and Petroff 1999]
New infestations originate from seeds and vegetative buds on roots. Farm operations and equipment, vehicles, recreational activities, and other human actions can transport seeds. Check and clean all equipment before moving from an infested to an uninfested area. Use fill material that is weed-free, particularly in uninfested areas. When moving sheep or cattle from infested to uninfested areas, hold them in corrals/pastures for 11 or 6 days respectively, to allow time for viable seeds to pass through their digestive tracts. Monitor and control Toadflax in the holding areas. Purchase weed-free seed or hay.
CHEMICAL CONTROL [Adapted from Lajeunesse
in Sheley and Petroff 1999]
Yellow Toadflax is difficult to control with herbicides due to its high genetic variability, waxy leaf surface, soil type, and biology. Herbicides must be applied using a surfactant because leaves have a waxy surface (cuticle) which acts as a protective barrier, hindering uptake. Herbicides are more likely to leach when applied to plants growing in sandy soils or soils low in organic matter, which could result in indirect negative impacts. Even where herbicides appear effective, long-term control may not be achieved and reinvasion from dormant seeds may occur. Where herbicides are effective, infestations should be treated annually for 3 years (Jacobs and Sing 2007). Herbicides are more effective when applied in the spring before blooming (to reduce seed production), during flowering, and/or during fall re-growth (Jacobs and Sing 2007).
In a Colorado study,
Picloram applied at 0.5 pounds per acre at the flowering stage at three different sites resulted in either 100, 69, or 35 percent control.
In a study
Picloram (1.12 kg ai/ha) mixed with
Fluroxypry (0.89 kg ai/ha) applied to just before blooming resulted in fair to good control.
Chlorsulfuron applied at 1.25 ounces per acre at flowering or in the fall found 84% control after one year.
Studies using
2,4-D,
2,4-DB,
MCPA, or
MCPB were ineffective. Spot treatments in low-till cropland using
Glyphosate,
Amitrole,
diquat or
Picloram have been used. Applying Glyphosate along with cultivation has been effective for reducing Yellow Toadflax for up to 2 years.
Additional study results can be found in the Natural Resources and Conservation Service pamphlet by Jacobs and Sing 2007.
MECHANICAL and PHYSICAL CONTROL [Adapted from Lajeunesse
in Sheley and Petroff 1999]
Hand-pulling can be effective for small infestations, particularly when soil is moist or where sandy. To delete the root reserves, pulling annually for 5-6 years and removing the lateral roots is necessary. To remove first-year seedlings, a site needs to be re-visited annually for 10-15 years.
Mowing is often not practical on most sites and is not very effective since a lot of growth occurs by rhizomes.
Cultivation: Sweep-type cultivators used from at least early June and repeated every 7-10 days can control Toadflax. To eradicate, 4-5 cultivations are required in the second year. However, inconsistent tillage can spread plants. Machinery should be thoroughly cleaned to prevent spreading root fragments.
Burning can remove standing biomass of Toadflax, but will also stimulate seed germination and root sprouting. Therefore, burning is not a recommended control method.
REVEGETATION [Adapted from Lajeunesse
in Sheley and Petroff 1999]
Management practices, seeding, and plantings that encourage growth of desirable plants and those well-adapted to the environment will increase competition against Yellow Toadflax seedlings and rosettes. Revegetation strategies can be effective since Yellow Toadflax seed production and viability are low. Revegetation or seeding should use several species that root at shallow, intermediate, and deep depths (least as deeply as Yellow Toadflax) in order to maximize competition for water, nutrients, and space. The mixture of species should provide active growth for as much of the year as possible, and include winter and summer annuals and shallow-rooted perennials. Desirable winter and summer annuals might compete well against Yellow Toadflax seedlings.
For information on revegetation species and seeding rates refer to Montana Plant Materials Technical Note 46, "Seeding Rates and Recommended Cultivars" and Extension Bulletin EB19 "Dryland Pasture species for Montana and Wyoming".
GRAZING MANAGEMENT [Adapted from Lajeunesse
in Sheley and Petroff 1999]
Cattle usually avoid Dalmatian and Yellow Toadflax, though casual browsing has been observed along with reports of mild poisoning. Sheep can consume Dalmatian Toadflax as a major food source and not show signs of poisoning.
The timing of grazing is important in developing and maintaining competitive, desirable plant communities. Overgrazing sites encourages Yellow Toadflax germination and growth. This is particularly true in the spring because seedlings can capture available soil moisture and other resources better than overgrazed plants.
When moving livestock from an infested to an uninfested area, hold cattle for 6 days and sheep for 11 days in corrals or small pastures until viable seeds have time to pass through their digestive tract. Monitor these areas for seedling establishment and provide control where seen. Avoid purchasing feed or seed that is contaminated by weeds.
BIOLOGICAL CONTROL [Sources Lajeunesse
in Sheley and Petroff 1999; Jacobs and Sing 2007.]
In general, it is recommended that at least 200 insects be established to create a sustainable population. Infestations should be at least 2 acres with sizeable populations. It may take 2-3 years for the insect population to establish.
As of 1998 6 insects have been approved and released in the U.S. and Canada for use on both Dalmatian and Yellow Toadflaxes. In Montana, none of these bio-control insects have proved to be highly effective on killing Yellow Toadflax foliage, but have reduced its fitness.
Toadflax Flower Beetle (
Brachypterolus pulicarius) is thought to have been accidentally introduced, and now occurs throughout North America. Adult beetles feed primarily on growing shoot tips and axillary buds, but can also feed on pollen, anthers, and ovaries. Larvae feed entirely on pollen, anthers, ovaries, and immature seeds.
Toadflax Seedhead Weevils (
Gymnaetron antirrhini and
Rhinusa neta) are thought to be accidentally introduced to North America. Rhinusa antirrhinin is more widely distributed. They impact seeds by stimulating the development of a gall, and their larvae feed on both deformed and normal seeds. R. neta larvae also feed on seeds, but without the development of a gall.
The timing of maturity between Brachypterolus pulicarius and Gymnaetron species can result in an interaction where B. pulicarius larvae predate upon the eggs of Gymnaetron species.
Toadflax Brocade Moth Calophasia lunula larvae feed on the lower leaves and stems of Yellow Toadflax. Their feeding can exhibit significant mortality to seedlings and young plants. However, pathogens within this insect constrains it from building a large enough population that is needed to significant impact Yellow Toadflax.
Root-mining Cosmet Moth Eteobalea intermediella has showed great promise for controlling Yellow Toadflax in studies, but for unexplained reasons this insect has not established well in North America.
Toadflax Stem-boring Weevil Mecinus janthinus was originally collected from Yellow Toadflax in its native range. While this biocontrol insect has impacted Dalmatian Toadflax populations very well, it has not affected Yellow Toadflax in western North America, and researchers do not know why. Researchers are now evaluating
M. heydeni for targeting Yellow Toadflax.
Useful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesStewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68. Jacobs, Jim, and Sharlene Sing. 2007. Ecology and Management of Yellow Toadflax [Linaria vulgaris (L.) Mill.].
Jacobs, Jim, and Sharlene Sing. 2007. Ecology and Management of Yellow Toadflax [Linaria vulgaris (L.) Mill.]. Koch, J., J. Strange, and P. Williams. 2012. Bumble bees of the western United States. Washington, DC: USDA Forest Service, Pollinator Partnership. 143 p.
Koch, J., J. Strange, and P. Williams. 2012. Bumble bees of the western United States. Washington, DC: USDA Forest Service, Pollinator Partnership. 143 p. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Macior, L.M. 1974. Pollination ecology of the Front Range of the Colorado Rocky Mountains. Melanderia 15: 1-59.
Macior, L.M. 1974. Pollination ecology of the Front Range of the Colorado Rocky Mountains. Melanderia 15: 1-59. Majerus, Mark, Joseph Scianna, and Jim Jacobs. 2013. Plant Materials Technical Note 46: Seeding Rates for Conservation Species for Montana. September. U.S. Department of Agriculture, Natural Resources and Conservation Service, Bozeman, Montana.
Majerus, Mark, Joseph Scianna, and Jim Jacobs. 2013. Plant Materials Technical Note 46: Seeding Rates for Conservation Species for Montana. September. U.S. Department of Agriculture, Natural Resources and Conservation Service, Bozeman, Montana. Miller-Struttmann, N.E. and C. Galen. 2014. High-altitude multi-taskers: bumble bee food plant use broadens along an altitudinal productivity gradient. Oecologia 176:1033-1045.
Miller-Struttmann, N.E. and C. Galen. 2014. High-altitude multi-taskers: bumble bee food plant use broadens along an altitudinal productivity gradient. Oecologia 176:1033-1045. Plath, O.E. 1934. Bumblebees and their ways. New York, NY: Macmillan Company. 201 p.
Plath, O.E. 1934. Bumblebees and their ways. New York, NY: Macmillan Company. 201 p. Robocker, W.C. 1970. Seed characteristics and seedling emergence of Dalmatian toadflax. Weed Scie. 18: 720-725.
Robocker, W.C. 1970. Seed characteristics and seedling emergence of Dalmatian toadflax. Weed Scie. 18: 720-725. Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon. Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79.
Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79. Williams, P., R. Thorp, L. Richardson, and S. Colla. 2014. Bumble Bees of North America. Princeton, NJ: Princeton University Press. 208 p.
Williams, P., R. Thorp, L. Richardson, and S. Colla. 2014. Bumble Bees of North America. Princeton, NJ: Princeton University Press. 208 p.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Breitenfeldt, T.A. 1995. Age-specific life table studies of potential strains or host races of Calophasia lunula (Lepidoptera: Noctuidae (Hufn.) reared on Dalmation toadflax, Linaria genistifolia ssp. dalmatica (L.) Maire and Petitmengen, or yellow toadflax, Linaria vulgaris (Mill.). M.Sc. Thesis. Bozeman, MT: Montana State University. 97 p.
Breitenfeldt, T.A. 1995. Age-specific life table studies of potential strains or host races of Calophasia lunula (Lepidoptera: Noctuidae (Hufn.) reared on Dalmation toadflax, Linaria genistifolia ssp. dalmatica (L.) Maire and Petitmengen, or yellow toadflax, Linaria vulgaris (Mill.). M.Sc. Thesis. Bozeman, MT: Montana State University. 97 p. Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp.
Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp. Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p.
Fogelsong, M.L. 1974. Effects of fluorides on Peromyscus maniculatus in Glacier National Park. M.Sc. Thesis. Bozeman, Montana: Montana State University. 52 p. Guenther, G.E. 1989. Ecological relationships of bitterbrush communities on the Mount Haggin Wildlife Management Area. M.Sc. Thesis. Bozeman, MT: Montana State University. 73 p.
Guenther, G.E. 1989. Ecological relationships of bitterbrush communities on the Mount Haggin Wildlife Management Area. M.Sc. Thesis. Bozeman, MT: Montana State University. 73 p. Holzwoth, Larry, Jeff Mosely, Dennis Cash, David Koch, and Kelly Crane. 2003. Dryland Pastures in Montana and Wyoming. Revised Fall. Extension Bulletin 19. Montana State University Extension, Bozeman, Montana.
Holzwoth, Larry, Jeff Mosely, Dennis Cash, David Koch, and Kelly Crane. 2003. Dryland Pastures in Montana and Wyoming. Revised Fall. Extension Bulletin 19. Montana State University Extension, Bozeman, Montana. Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. McDermott, G.J. 1991. Determination of host races in three insect species attacking Dalmation toadflax and yellow toadflax in North America. M.Sc. Thesis.Bozeman, MT: Montana State University. 43 p.
McDermott, G.J. 1991. Determination of host races in three insect species attacking Dalmation toadflax and yellow toadflax in North America. M.Sc. Thesis.Bozeman, MT: Montana State University. 43 p. Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park.
Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park. Simanonok, M. 2018. Plant-pollinator network assembly after wildfire. Ph.D. Dissertation. Bozeman, MT: Montana State University. 123 p.
Simanonok, M. 2018. Plant-pollinator network assembly after wildfire. Ph.D. Dissertation. Bozeman, MT: Montana State University. 123 p. Simanonok, M.P. and L.A. Burkle. 2019. Nesting success of wood-cavity-nesting bees declines with increasing time since wildfire. Ecology and Evolution 9:12436-12445.
Simanonok, M.P. and L.A. Burkle. 2019. Nesting success of wood-cavity-nesting bees declines with increasing time since wildfire. Ecology and Evolution 9:12436-12445. Tosevski, I., S.E. Sing, R. De Clerk-Floate, A. McClay, J. Jovic, and A. Gassmann. 2018. Twenty-five years after: post-introduction association of Mecinus janthinus s.l. with invasive host Toadflaxes Linaria vulgaris and Linaria dalmatica in North America. Annals of Applied Biology 173:16-34.
Tosevski, I., S.E. Sing, R. De Clerk-Floate, A. McClay, J. Jovic, and A. Gassmann. 2018. Twenty-five years after: post-introduction association of Mecinus janthinus s.l. with invasive host Toadflaxes Linaria vulgaris and Linaria dalmatica in North America. Annals of Applied Biology 173:16-34. Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp.
Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp.
- Web Search Engines for Articles on "Yellow Toadflax"





