View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Bohemian Knotweed - Polygonum x bohemicum
Other Names:
Fallopia ×bohemica, Polygonum cuspidatum × Polygonum sachalinense, Reynoutria ×bohemica
State Rank Reason (see State Rank above)
Polygonum xbohemicum(Fallopia xbohemica) is a hybrid between the Japanese Polygonum cuspidatum and Giant KnotweedsPolygonum sachalinense. It is part of the Japanese Knotweed Complex. A conservation status rank is not applicable (SNA) because the plant is an exotic (non-native) in Montana that is not a suitable target for conservation activities.
General Description
PLANTS: Large, rhizomatous, herbaceous perennials with erect, branched stems that reach to heights of 2-5 meters. Sources: FNA 2005; Parkinson and Mangold 2017.
LEAVES: Petiolate and alternately arranged. Blades are ovate, 5-20 cm long with acute tips and a slightly heart-shaped (cordate) base. Stipules occur at leaf nodes and are brown, glabrous, and 4-6(10) mm long. Sources: FNA 2005; Parkinson and Mangold 2017.
INFLORESCENCE: Flowers arranged in panicles at leaf axils or are terminal on branches. The greenish-white to creamy-white flowers appear perfect (with stamens and pistils) but may function unisexually or are just female (with pistils). Each flower with pedicel (stem) is 4.5–6.5 mm long with a tubular base. The petals and sepals look-alike (tepals) and are greenish-white to creamy-white. Fruit is an achene that is shiny, smooth, dark brown, 2.6–3.2 mm long, 3-sided, and enclosed by an inflated perianth (tepals). Sources: FNA 2005; Parkinson and Mangold 2017.
Phenology
Flowers July through October (FNA 2005).
Diagnostic Characteristics
The
Japanese Knotweed complex includes Japanese Knotweed, Giant Knotweed, and Bohemian Knotweed which is a hybrid between the Japanese and Giant Knotweeds. Japanese, Giant, and Bohemian plants are often mis-identified with each other. A strong hand-lens is required. Schutter Diagnostic Laboratory at Montana State University, Bozeman can assist in identifying good quality plant specimens.
Plants in the
Japanese Knotweed complex exhibit flowers with fringed stigmas and erect, hollow stems that grow in clumps, resembling bamboo. Unlike bamboo, leaves of the knotweed complex are ovate in shape and have brown papery or membranous sheaths at the leaf nodes. Other
Polygonum species may be commonly called
Knotweed or
Smartweed, but are either less than 1 meter tall or are vines or have mostly basal leaves and few stem leaves, and species in the “complex” only have hollow stems.
Japanese Knotweed -
Polygonum cuspidatum, Fallopia japonica, or
Reynoutria japonica* Leaf blades are 1-4 inches (3-10 cm) long.
* The leaf tip is abrupt and also acuminate.
* Veins on the lower surface of leaf are minutely scabrous (roughened) with swollen cells or knobs. Hairs if present are short, less than 0.1mm tall, unicellular, and with blunt tips.
* Mid-branch leaves with a leaf base that is flat where it joins the petiole - truncate to rarely slightly cuneate in shape.
* Inflorescence is usually longer than the subtending mid-branch leaf.
* Plants are approximately 5-8 feet (1.5-2.5 meters) tall, tending to be shortest in the complex.
* Plants usually don’t produce seeds.
Giant Knotweed -
Polygonum sachalinense, Fallopia sachalinensis, or
Reynoutria sachalinensis* Leaf blades are 7.8-16 inches (20-40 cm) long
* The leaf is more evenly tapered to a blunt or acute tip.
* Veins on the lower surface of leaf have multicellular hairs of 0.2-0.6 mm tall.
* Mid-branch leaves with a leaf base that is deeply heart-shaped where it joins the petiole - cordate in shape.
* Inflorescence is much shorter than the subtending mid-branch leaf.
* Plants are approximately 9.9-19.8 feet (3-6 meters) tall and branches sparingly. It is the tallest in the complex.
* Plants usually produce seeds.
Bohemian Knotweed -
Fallopia x
bohemica, a hybrid
* Leaf blades are 2-12 inches (5-30 cm) long. Largest mid-stem leaves are usually greater than 20 cm long.
* The leaf tip is usually not abrupt (not cuspidate), but is acuminate.
* Veins on the lower surface of leaf are obscure (puberulent), not roughened, short, and acute at the tip.
* Mid-stem leaves with a leaf base that is flat to slightly heart-shaped where it joins the petiole – truncate to slightly cordate in shape.
* Inflorescence is either shorter or longer than the subtending mid-branch leaf.
* Plants are approximately 6.6-16.5 feet (2-5 meters) tall.
* Plants occasionally produce seeds.
Sources: Zika and Jacobson 2003; Flora of North America 2005; Lesica et al. 2012; Parkinson and Mangold 2017.
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Bohemian Knotweed was first described in Europe (Parkinson and Mangold 2017). It is unknown if Bohemian Knotweed arrived in North America from Europe or if it developed in North America from crosses between Giant and Japanese Knotweeds (Parkinson and Mangold 2017). In England this hybrid was grown in gardens since 1872, but the parent origins were not determined until recently (Zika and Jacobson 2003). In fact, it was not until 1983 that the hybrid was recognized, studied, and named by J. Chrtek and A. Chrtkova as Reynoutria xbohemica (Zika and Jacobson 2003). In reviewing North American herbarium specimens and botanical illustrations from literature dated from 1964 to 1993, Bohemian Knotweed has been unrecognized and mis-labelled as Polygonum cuspidatum (Zika and Jacobson 2003). The work by Zika and Jacobson (2003) has clarified that Bohemian Knotweed was cultivated in North American for garden ornamentals from which it has vegetatively spread into natural areas.
Herbarium specimens collected in 2004 from Mineral and Sanders County confirmed its presence in Montana. See the Consortium of Pacific Northwest Herbaria portal (http://www.pnwherbaria.org).
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
Observations in Montana Natural Heritage Program Database
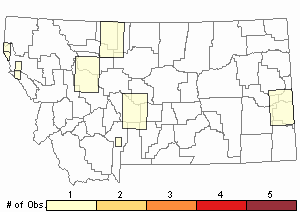
Number of Observations: 13
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
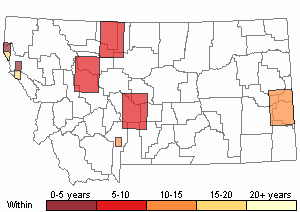
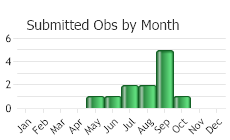
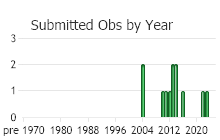
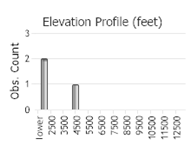 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
In Montana it grows along Noxon Reservoir and the St. Regis River (Consortium of Pacific Northwest Herbaria;
https://www.pnwherbaria.org).
Ecology
POLLINATORS The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus bifarius,
Bombus flavifrons,
Bombus frigidus,
Bombus melanopygus,
Bombus sylvicola,
Bombus occidentalis,
Bombus pensylvanicus,
Bombus insularis, and
Bombus kirbiellus (Macior 1974, Colla and Dumesh 2010).
Reproductive Characteristics
Reproduction is primarily vegetative by rhizomes, and also stem fragments (Parkinson and Mangold 2017). Seed production is occasional (Parkinson and Mangold 2017). Bohemian Knotweed is believed to have better regeneration abilities than its parental species, thus is can spread without the presence of Japanese and Giant Knapweeds (Parkinson and Mangold 2017).
LIFE CYCLE [Adapted from Parkinson and Mangold 2017]
From mid-spring to late summer new shoots emerge from existing root crowns and rhizomes. Shoots are fleshy, pointed at the tip, slender, not hollow, and may appear like asparagus shoots. Growth is rapid. Plants are not cold-tolerant. Upon cold temperatures above ground foliage can die. From July to October flowers develop (FNA 2005). Flowers often have stamens and pistils or are all female (Parkinson and Mangold 2017). The pollen of Giant Knotweed can fertilize the flowers of female Japanese or Bohemian Knotweed plants allowing them to produce seed. Likewise fertile pollen from a Bohemian Knotweed plant may backcross to fertilize a female Japanese Knotweed plant. It is assumed that seed may also develops 2-3 weeks after flowering. Although foliage dies with cold temperatures, dead stems (canes) and a few fruits may remain visible during the winter.
Growth and dispersal occurs primarily at nodes on the rhizomes, but can also occur from fragmented stems. It is assumed that small fragmented rhizomes can start new colonies. Rhizomes become fragmented when plants are disturbed, particularly when adjacent soil is excavated, or plants are moved. Rhizomes can also break off from plants growing along streams, float downstream, and upon landing in a suitable place will regenerate. Likewise, stem fragments and cuttings can develop roots and shoots upon landing on moist soil or grass. Once growth is initiated, whether by above-ground stems or below-ground rhizomes, development of lateral roots and new shoots is rapid.
Management
Proper identification, early detection, and control of Bohemian Knotweed in Montana is key to preventing its establishment. It is assumed that efforts to control Bohemian Knotweed will require a combination of techniques for many years (Parkinson and Mangold 2017).
It is assumed that the management techniques used for Japanese Knotweed will apply successfully to Bohemian Knapweed.
PREVENTION [Adapted from Parkinson and Mangold 2017.]
Identify and suppress any found populations. Do not spread soil from infested areas because the soil will contain root fragments that can re-generate in other areas.
MECHANICAL and PHYSICAL CONTROLS [Adapted from Parkinson and Mangold 2017.]
Placing a
heavy black plastic tarp tightly to the ground for more than one year can suppress plants. This is recommended for small infestations. However, rhizomes can go dormant for 20 years and frequently monitoring is required to document presence/absence.
Hand-pulling or
digging is effective for small populations where plants are young. Plants should be pulled in moist soil and twice each month to remove new sprouts.
Stem Cutting is labor intensive, but effective. Cutting should occur 3 times each year for consecutive, multiple years, in order to reduce the reserves in the rhizomes. Further the last cutting in a year should occur before plants lose their leaves (which is near the onset of winter).
Mowing is effective if done close to the ground and repeated when plants reach 6 inches tall. Mowing should occur through the entire growing season.
Tilling alone is not recommended because it breaks rhizomes and encourages re-sprouting. However, it can be effective to stimulate leaf growth prior to a herbicide application.
Revegetation in combination with other techniques is recommended. On its own Revegetating sites will not be enough. Sites that appear to be eradicated or greatly suppressed, should be planted with competitive shrubs that are appropriate for the site. Competitive grasses can also be used in combination with shrubs. Potential shrubs could include species of willow (
Salix spp.), Blue Elderberry (
Sambucus cerulean), Red/Black Elderberry (
Sambucus racemosa), Green Alder (
Alnus viridis), Speckled Alder (
Alnus incana). Potential grasses to plant with shrubs could include: Streambank Wheatgrass (
Elymus lanceolatus) or great Basin Wildrye (
Elymus cinereus).
CHEMICAL CONTROL [Adapted from Parkinson and Mangold 2017.]
Herbicides are effective, especially when properly managed with other tactics. The herbicide type and concentration, timing of chemical control, soil properties, and other factors will determine its effectiveness and impact to non-target species. Strict adherence to application requirements defined on the herbicide label will reduce risks to human and environmental health. Many herbicides must be applied by applicators with an Aquatic Pest Control license. Consult your County Extension Agent and/or Weed District for more information on herbicidal control. Chemical information is also available at
Greenbook.
Stem injection is commonly used and involves using a hand-operated injection device that delivers repeated, pre-measured doses. Prior to injection, a hole must be made using an awl or similar tool. All stems must be treated.
Glyphosate (0.17 ounce or 5 mL) injected into the hollow stem between the second and third node or about six inches above the ground has been used successfully.
Glyphosate and 2,4-D (0.2 ounce or 6 mL) injected into the hollow stem between the second and third node has been used successfully.
Foliar applications have been used successfully.
Aminopyralid broadcast sprayed at 7-14 ounces per acre when plants are 3-4 feet tall has been used successfully.
Imazapyr and
Metsulfuron Methyl chemicals are not selective and kill all plants and should only be used in non-crop sites. These chemicals have been broadcast sprayed at 25 ounces per acre after plants emerge.
Imazapyr can also be applied at 4-6 pints per acre when plants are actively growing and a surfactant is recommended. In and around water,
Imazapyr can be used on actively growing foliate at 3-4 pints per acre and use an adjuvant that is approved for aquatic use.
GRAZING CONTROLs [Adapted from Parkinson and Mangold 2017.]
Young shoots of Japanese Knotweed are palatable to sheep, goats, cattle, and horses. Grazing does not kill plants, but does weaken them. When grazing pressure is high, the establishment and growth of Japanese Knotweed is reduced.
BIOLOGICAL CONTROL [Adapted from Parkinson and Mangold 2017.]
No bio-control insects, pathogens, or fungi have been approved there are candidates being tested and screened for use in the U.S.
Useful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesStewardship Responsibility
Threats or Limiting Factors
It is assumed that the monotypic, dense stands of Bohemian Knotweed impact the environment similar to that by Japanese Knotweed. Research with Giant Knotweed has not received as much attention. Therefore, Giant Knot is assumed to alter water quality which impacts food chains and fisheries, to expose soils along streambanks to accelerated erosion during winter.
Its growth is fast and aggressive; therefore, it is assumed that it could damage cement foundations, pathways, and walls, and interfere with transportation safety.
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68. Flora of North America Editorial Committee. 2005. Flora of North America North of Mexico. Volume 5. Magnoliophyta: Caryophyllidae: Caryophyllales, Polygonales, and Plumbaginales. New York, NY: Oxford Univ. Press. vii + 656 pp.
Flora of North America Editorial Committee. 2005. Flora of North America North of Mexico. Volume 5. Magnoliophyta: Caryophyllidae: Caryophyllales, Polygonales, and Plumbaginales. New York, NY: Oxford Univ. Press. vii + 656 pp. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Macior, L.M. 1974. Pollination ecology of the Front Range of the Colorado Rocky Mountains. Melanderia 15: 1-59.
Macior, L.M. 1974. Pollination ecology of the Front Range of the Colorado Rocky Mountains. Melanderia 15: 1-59. Parkinson, Hilary, and Jane Mangold. 2017. Biology, Ecology, and Management of the Knotweed Complex (Fallopia and Persicaria spp.). Revised August. EB0196. Montana State University Extension, Bozeman, Montana.
Parkinson, Hilary, and Jane Mangold. 2017. Biology, Ecology, and Management of the Knotweed Complex (Fallopia and Persicaria spp.). Revised August. EB0196. Montana State University Extension, Bozeman, Montana. Zika, Peter F. and Arthur L. Jacobson. 2003. An Overlooked Hybrid Japanese Knotweed (Polygonum cuspidatum x sachalinense; Polygonaceae) in North America. Rhodora, Volume 105, Number 922: 143-152.
Zika, Peter F. and Arthur L. Jacobson. 2003. An Overlooked Hybrid Japanese Knotweed (Polygonum cuspidatum x sachalinense; Polygonaceae) in North America. Rhodora, Volume 105, Number 922: 143-152.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
- Web Search Engines for Articles on "Bohemian Knotweed"





