View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Japanese Knotweed - Polygonum cuspidatum
Other Names:
Fallopia japonica, Reynoutria japonica, Reynoutria japonica var. japonica
State Rank Reason (see State Rank above)
Polygonum cuspidatum (Fallopia japonica) is native to Japan, China, Korea, and Taiwan (Parkinson and Mangold 2017). It is part of the Japanese Knotweed Complex. A conservation status rank is not applicable (SNA) because the plant is an exotic (non-native) in Montana that is not a suitable target for conservation activities.
NOTE: There is a discrepancy between the number of observations and counties represented in the herbaria (Consortium of Pacific Northwest Herbaria; www.pnwherbaria.org) versus the Montana Natural Heritage Program databases, which indicates a problem. It is important to document new occurrences with nice quality plant specimens of which some should be deposited in our State herbaria (University of Montana, Montana State University, Montana State University-Billings). Depositing specimens in the herbaria allows identifications to be confirmed, provides a central location for educating and sharing information, and allows specimens to be studied for genetics, morphology, and ecology.
General Description
PLANTS: Large, rhizomatous, herbaceous perennials with erect, branched stems that reach to 2 meters (6 feet) tall in Montana. In other states they may reach to almost 6 meters (19 feet) in height. Sources: Parkinson and Mangold 2017; Lesica et al. 2012.
LEAVES: Petiolate and alternately arranged. Blades are ovate, 8–20 cm long with pointed tips and a truncate base. Stipules occur at leaf nodes and are brown, glabrous, and 4–6 mm long. Source: Lesica et al. 2012.
INFLORESCENCE: Flowers arranged in panicles at leaf axils. The greenish-white to creamy-white flowers appear perfect with stamens and pistils, but functionally are unisexual. Each flower is 5–7 mm long with a tubular base and pedicels (stems) of 3–5 mm long. The petals and sepals look-alike (tepals) and are white to pink. Fruit is an achene that is shiny-brown, 2–4 mm long, 3-sided, and enclosed by an inflated perianth (tepals). Source: FNA 2005; Lesica et al. 2012.
Phenology
Flowering occurs in August to September and fruits/seeds develop in September (Parkinson and Mangold 2017).
Diagnostic Characteristics
The
Japanese Knotweed complex includes Japanese Knotweed, Giant Knotweed, and Bohemian Knotweed which is a hybrid between the Japanese and Giant Knotweeds. Japanese, Giant, and Bohemian plants are often mis-identified with each other. A strong hand-lens is required. Schutter Diagnostic Laboratory at Montana State University, Bozeman can assist in identifying good quality plant specimens.
Plants in the
Japanese Knotweed complex exhibit flowers with fringed stigmas and erect, hollow stems that grow in clumps, resembling bamboo. Unlike bamboo, leaves of the knotweed complex are ovate in shape and have brown papery or membranous sheaths at the leaf nodes. Other
Polygonum species may be commonly called
Knotweed or
Smartweed, but are either less than 1 meter tall or are vines or have mostly basal leaves and few stem leaves, and species in the “complex” only have hollow stems.
Japanese Knotweed -
Polygonum cuspidatum, Fallopia japonica, or
Reynoutria japonica* Leaf blades are 1-4 inches (3-10 cm) long.
* The leaf tip is abrupt and also acuminate.
* Veins on the lower surface of leaf are minutely scabrous (roughened) with swollen cells or knobs. Hairs if present are short, less than 0.1mm tall, unicellular, and with blunt tips.
* Mid-branch leaves with a leaf base that is flat where it joins the petiole - truncate to rarely slightly cuneate in shape.
* Inflorescence is usually longer than the subtending mid-branch leaf.
* Plants are approximately 5-8 feet (1.5-2.5 meters) tall, tending to be shortest in the complex.
* Plants usually don’t produce seeds.
Giant Knotweed -
Polygonum sachalinense, Fallopia sachalinensis, or
Reynoutria sachalinensis* Leaf blades are 7.8-16 inches (20-40 cm) long.
* The leaf is more evenly tapered to a blunt or acute tip.
* Veins on the lower surface of leaf have multicellular hairs of 0.2-0.6 mm tall.
* Mid-branch leaves with a leaf base that is deeply heart-shaped where it joins the petiole - cordate in shape.
* Inflorescence is much shorter than the subtending mid-branch leaf.
* Plants are approximately 9.9-19.8 feet (3-6 meters) tall and branches sparingly. It is the tallest in the complex.
* Plants usually produce seeds.
Bohemian Knotweed -
Fallopia x
bohemica, a hybrid
* Leaf blades are 2-12 inches (5-30 cm) long. Largest mid-stem leaves are usually greater than 20 cm long.
* The leaf tip is usually not abrupt (cuspidate), but is acuminate.
* Veins on the lower surface of leaf are obscure (puberulent), not roughened, short, and acute at the tip.
* Mid-stem leaves with a leaf base that is flat to slightly heart-shaped where it joins the petiole – truncate to slightly cordate in shape.
* Inflorescence is either shorter or longer than the subtending mid-branch leaf.
* Plants are approximately 6.6-16.5 feet (2-5 meters) tall.
* Plants occasionally produce seeds.
Sources: Zika and Jacobson 2003; Flora of North America 2005; Lesica et al. 2012; Parkinson and Mangold 2017.
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Japanese Knotweed is native to Japan, China, Korea, and Taiwan (Parkinson and Mangold 2017). Plants were introduced into North America in the late 1800s as an ornamental (Parkinson and Mangold 2017). Plants have escaped and are found from British Columbia to Quebec and south to most of the U.S. (Lesica 2012 et. al.).
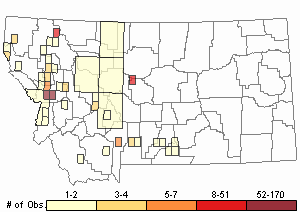
In Montana herbarium specimens demonstrate that it was present in landscaped yards in Missoula County since 1977, but the plant was not documented with a herbarium specimen until 1997 when it was found to have colonized a nearby ravine. An earlier specimen documents it was growing at a fishing access site in Sweet Grass County in 1995. Since then herbarium specimens have documented it in Sanders and Missoula Counties. Other observations are finding it in many counties of western and central Montana where needed specimens have not been provided to our State Herbaria [Refer to OBSERVATIONS IN MONTANA NATURAL HERITAGE PROGRAM DATABASE].
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
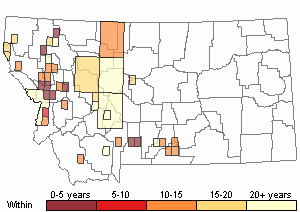
Observations in Montana Natural Heritage Program Database
Number of Observations: 412
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
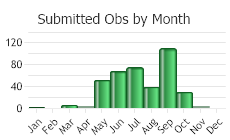
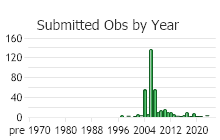
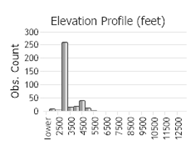 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
In its native habitat it is an early successional plant and can colonize volcanic slopes (Parkinson and Mangold 2017). In Europe and North America it was planted into yards and managed landscapes, but has escaped into moist habitats that include riverbanks, canals, wetlands, lakeshores, utility right-of-ways, strip-mining areas, and roadways (Parkinson and Mangold 2017). In Montana it grows in gardens and lawns and along streambanks and reservoirs in valleys (Lesica et al. 2012).
Japanese Knotweed is considered shade intolerant and has been found in soil with pH ranging from 4.5 to 7.4 (Parkinson and Mangold 2017). Plants are not limited by soil type and can establish, grow, and have good survival rates in nutrient-poor to nutrient-rich soils. In the Cascade Range, Japanese Knotweed has been found in open canopies with Black Poplar (Populus nigra), Red Alder (Alnus rubra), and Douglas-fir (Pseudotsuga menziesii) which implies that open-canopy forests in Montana could be vulnerable (Parkinson and Mangold 2017).
Ecology
Monotypic, dense stands of Japanese Knotweed can alter water quality which can impact the food chains and fisheries(Parkinson and Mangold 2017). Monotypic, dense stands along waterways expose soils in the winter time increasing their vulnerability to erosion.
POLLINATORS The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus bifarius,
Bombus flavifrons,
Bombus frigidus,
Bombus melanopygus,
Bombus sylvicola,
Bombus occidentalis,
Bombus pensylvanicus,
Bombus insularis, and
Bombus kirbiellus (Macior 1974, Colla and Dumesh 2010).
Reproductive Characteristics
Reproduction is primarily vegetative by rhizomes, and also stem fragments (Parkinson and Mangold 2017). Seed production is rare (Parkinson and Mangold 2017). Hybrids (Fallopia xbohemica) can also form where Polygonum cuspidatum and Polygonum sachalinense occur together.
FLOWERS [Adapted from Lesica et al. 2012]
Flowers appear perfect with stamens and pistils, but functionally are unisexual. Each flower is 5–7 mm long with a tubular base and pedicels (stems) of 3–5 mm long. The petals and sepals look-alike (tepals) and are white to pink.
FRUITS [Adapted from Lesica et al. 2012]
The fruit is an achene. Achenes are shiny-brown, 2–4 mm long, 3-sided, and enclosed by an inflated perianth (tepals).
LIFE CYCLE [Adapted from Parkinson and Mangold 2017]
From mid-spring to late summer new shoots emerge from existing root crowns and rhizomes. Shoots are fleshy, pointed at the tip, slender, not hollow, and may appear like asparagus shoots. Growth is rapid. Plants are not cold-tolerant. Upon cold temperatures above ground foliage can die. From August to September flowers develop. Flowers often have stamens and pistils, but functionally are unisexual. Most flowers function as female (Parkinson and Mangold 2017), but Montana herbarium specimens mostly represent male plants (Lesica et al. 2012). Occasionally mixed-sex populations occur and in September fruits may appear. When seed is produced it often develops 2-3 weeks after flowering. Seeds that remain on the stem longer have a higher germination rate. A study in Pennsylvania found Japanese Knotweed seeds collected in early September had less than 10% germination rates while seeds collected 4 weeks later had up to 90% germination rates (Bram and McNair 2004). Although foliage dies with cold temperatures, dead stems (canes) and a few fruits may remain visible during the winter.
Growth and dispersal occurs primarily at nodes on the rhizomes, but can also occur from fragmented stems. Fragmented rhizomes as small as 0.02 pounds (7 grams) can start new colonies. Rhizomes become fragmented when plants are disturbed, particularly when adjacent soil is excavated, or plants are moved. Rhizomes can also break off from plants growing along streams, float downstream, and upon landing in a suitable place will regenerate. Likewise, stem fragments and cuttings can develop roots and shoots upon landing on moist soil or grass. Once growth is initiated, whether by above-ground stems or below-ground rhizomes, development of lateral roots and new shoots is rapid.
Management
Proper identification, early detection, and control of Japenese Knotweed in previously non-infested sites is the key to preventing establishment of new colonies in Montana. Plants are found scattered in Montana, but have not yet developed impenetrable patches along our roadways and rivers; therefore, we must prevent spread while suppressing and eradicating existing infestations. Efforts to control Japanese Knotweed will require a combination of techniques for many years (Parkinson and Mangold 2017). Upon eradication or control, a 60-foot swatch around the population should be monitored yearly to determine if growth resumes.
PREVENTION [Adapted from Parkinson and Mangold 2017.]
Identify and suppress existing patches. Do not spread soil from infested areas because the soil will contain root fragments that can re-generate in other areas.
MECHANICAL and PHYSICAL CONTROLS [Adapted from Parkinson and Mangold 2017.]
Placing a
heavy black plastic tarp tightly to the ground for more than one year can suppress plants. This is recommended for small infestations. However, rhizomes can go dormant for 20 years and frequently monitoring is required to document presence/absence.
Hand-pulling or
digging is effective for small populations where plants are young. Plants should be pulled in moist soil and twice each month to remove new sprouts.
Stem Cutting is labor intensive, but effective. Cutting should occur 3 times each year for consecutive, multiple years, in order to reduce the reserves in the rhizomes. Further the last cutting in a year should occur before plants lose their leaves (which is near the onset of winter).
Mowing is effective if done close to the ground and repeated when plants reach 6 inches tall. Mowing should occur through the entire growing season.
Tilling alone is not recommended because it breaks rhizomes and encourages re-sprouting. However, it can be effective to stimulate leaf growth prior to a herbicide application.
Revegetation in combination with other techniques is recommended. On its own Revegetating sites will not be enough. Sites that appear to be eradicated or greatly suppressed, should be planted with competitive shrubs that are appropriate for the site. Competitive grasses can also be used in combination with shrubs. Potential shrubs could include species of willow (
Salix spp.), Blue Elderberry (
Sambucus cerulean), Red/Black Elderberry (
Sambucus racemosa), Green Alder (
Alnus viridis), Speckled Alder (
Alnus incana). Potential grasses to plant with shrubs could include: Streambank Wheatgrass (
Elymus lanceolatus) or great Basin Wildrye (
Elymus cinereus).
CHEMICAL CONTROL [Adapted from Parkinson and Mangold 2017.]
Herbicides are effective, especially when properly managed with other tactics. The herbicide type and concentration, timing of chemical control, soil properties, and other factors will determine its effectiveness and impact to non-target species. Strict adherence to application requirements defined on the herbicide label will reduce risks to human and environmental health. Many herbicides must be applied by applicators with an Aquatic Pest Control license. Consult your County Extension Agent and/or Weed District for more information on herbicidal control. Chemical information is also available at
Greenbook.
Stem injection is commonly used and involves using a hand-operated injection device that delivers repeated, pre-measured doses. Prior to injection, a hole must be made using an awl or similar tool. All stems must be treated.
Glyphosate (0.17 ounce or 5 mL) injected into the hollow stem between the second and third node or about six inches above the ground has been used successfully.
Glyphosate and 2,4-D (0.2 ounce or 6 mL) injected into the hollow stem between the second and third node has been used successfully.
Foliar applications have been used successfully.
Aminopyralid broadcast sprayed at 7-14 ounces per acre when plants are 3-4 feet tall has been used successfully.
Imazapyr and
Metsulfuron Methyl chemicals are not selective and kill all plants and should only be used in non-crop sites. These chemicals have been broadcast sprayed at 25 ounces per acre after plants emerge.
Imazapyr can also be applied at 4-6 pints per acre when plants are actively growing and a surfactant is recommended. In and around water,
Imazapyr can be used on actively growing foliate at 3-4 pints per acre and use an adjuvant that is approved for aquatic use.
GRAZING CONTROLs [Adapted from Parkinson and Mangold 2017.]
Young shoots are palatable to sheep, goats, cattle, and horses. Grazing does not kill plants, but does weaken them. When grazing pressure is high, the establishment and growth of Japanese Knotweed is reduced.
BIOLOGICAL CONTROL [Adapted from Parkinson and Mangold 2017.]
No bio-control insects, pathogens, or fungi have been approved there are candidates being tested and screened for use in the U.S.
Useful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesStewardship Responsibility
Threats or Limiting Factors
Monotypic, dense stands of Japanese Knotweed can form impenetrable walls which alter access to our waterways (Parkinson and Mangold 2017). They also alter water quality which impacts food chains and fisheries. Monotypic, dense stands along waterways expose soils in the winter time increasing their vulnerability to erosion (Parkinson and Mangold 2017), that under the cover of our native habitats would not have been ecologically normal.
Plants have also been found to push through concrete, displacing foundations, walls, walkways, and paved drainages (Parkinson and Mangold 2017). In England, foundations built in heavily infested areas require heavy duty barriiers warranteed to 50-years (Parkinson and Mangold 2017). In the eastern U.S., the growth of Japanese Knotweed has reduced roadway sight distances, blocked road signs, and damaged pavement (Parkinson and Mangold 2017).
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Bram, M.R. and J.N. McNair. 2004. Seed germinability and its seasonal onset of Japanese Knotweed (Polygonum cuspidatum). Weed Science, 52(5): 759-767.
Bram, M.R. and J.N. McNair. 2004. Seed germinability and its seasonal onset of Japanese Knotweed (Polygonum cuspidatum). Weed Science, 52(5): 759-767. Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68. Flora of North America Editorial Committee. 2005. Flora of North America North of Mexico. Volume 5. Magnoliophyta: Caryophyllidae: Caryophyllales, Polygonales, and Plumbaginales. New York, NY: Oxford Univ. Press. vii + 656 pp.
Flora of North America Editorial Committee. 2005. Flora of North America North of Mexico. Volume 5. Magnoliophyta: Caryophyllidae: Caryophyllales, Polygonales, and Plumbaginales. New York, NY: Oxford Univ. Press. vii + 656 pp. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Macior, L.M. 1974. Pollination ecology of the Front Range of the Colorado Rocky Mountains. Melanderia 15: 1-59.
Macior, L.M. 1974. Pollination ecology of the Front Range of the Colorado Rocky Mountains. Melanderia 15: 1-59. Parkinson, Hilary, and Jane Mangold. 2017. Biology, Ecology, and Management of the Knotweed Complex (Fallopia and Persicaria spp.). Revised August. EB0196. Montana State University Extension, Bozeman, Montana.
Parkinson, Hilary, and Jane Mangold. 2017. Biology, Ecology, and Management of the Knotweed Complex (Fallopia and Persicaria spp.). Revised August. EB0196. Montana State University Extension, Bozeman, Montana.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Zika, Peter F. and Arthur L. Jacobson. 2003. An Overlooked Hybrid Japanese Knotweed (Polygonum cuspidatum x sachalinense; Polygonaceae) in North America. Rhodora, Volume 105, Number 922: 143-152.
Zika, Peter F. and Arthur L. Jacobson. 2003. An Overlooked Hybrid Japanese Knotweed (Polygonum cuspidatum x sachalinense; Polygonaceae) in North America. Rhodora, Volume 105, Number 922: 143-152.
- Web Search Engines for Articles on "Japanese Knotweed"





