View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Common Tansy - Tanacetum vulgare
Other Names:
Golden Buttons
State Rank Reason (see State Rank above)
Tanacetum vulgare is a plant native to temperature portions of Eurasia and has been introduced into North America (Watson in FNA 2006). A conservation status rank is not applicable (SNA) because the plant is an exotic (non-native) in Montana that is not a suitable target for conservation activities.
General Description
PLANTS: Rhizomatous, perennial forbs with single, erect stems that grow 40-120 cm tall. Plants have few hairs (glabrate), milky sap, and ‘fern-like’ leaves. Source: Jacobs 2008; Lesica et al. 2012.
LEAVES: Basal leaves wither early. Stem leaves are alternately arranged and petiolate but become sessile upwards. Leaf blades are about 5-15 cm long, pinnately divided into lanceolate, serrate t sharply lobed leaflets. Leaves are glabrate and gland-dotted (punctate). The main stem between leaflets (rachis) is somewhat winged. Sources: Watson in FNA 2006; Lesica et al. 2012.
INFLORESCENCE: Flat-topped corymbiform. 20-200 yellow, rayless, button-like flowers are arranged in flat-topped, compact clusters. Flower heads have an involucre of 5-10 mm across, composed of subequal-sized bracts forming 2-3 rows. Flowerheads lack ray florets (lack petals) and are composed of only yellow disc florets, 1-3 mm tall. The involucre is composed of green, overlapping bracts that have dry, thin, membranous, and translucent margins and tips. The pappus is either crown-like or absent. Sources: Watson in FNA 2006; Lesica et al. 2012.
The genus Tanacetum is authored by Carl Linnaeus and is derived from the word athanotos which implies ‘immortality’ and references its uses in medicin and preservation. This plant was also called tanazetum or athanacetum in medieval Latin, and the Old French changed it to tanesie which sounds similar to ‘tansy’.
The specific epithet of vulgare is from Latin meaning ‘ordinary’ or ‘common’. Sources: Watson in FNA 2006; Jacobs 2008.
Phenology
Flowering July through September (Watson in FNA 2006).
Diagnostic Characteristics
Common Tansy and Tansy Ragwort are often confused with one another.
Common Tansy –
Tanacetum vulgare, exotic and Noxious:
* Yellow flowers lack petals. Flowers have yellow disc florets and lack ray florets.
* Leaves pinnately deeply divided into equal-sized, sharp-toothed lobes.
* Plants have few hairs and leaves are gland-dotted.
* Crushed leaves have a strong menthol- or camphor-like smell from volatile oils.
Tansy Ragwort –
Senecio jacobaea, exotic and Noxious:
* A biennial to short-lived perennial – pull a mature plant to find a dark-colored taproot.
* Stem leaves similar in size from base to top.
* Flowers with showy, yellow petals. Flower have both yellow ray and disc florets.
* Leaves pinnately deeply-divided into lobes that are shallowly divided.
* Lobe tips of leaves are rounded (not pointed).
* Plants have hairs, often cobwebby hairs when young leaves
Montana has 2 other
Tanacetum species, both exotic:
Coastmary –
Tanacetum balsamita, exotic:
* Yellow flowers lack petals. Flowers have yellow disc florets and lack ray florets.
* Leaves are simple and not lobed.
Feverfew –
Tanacetum parthenium, exotic:
* Flowers have white petals (ray florets) and yellow centers (disc florets).
* Plants are minutely hairy.
* Leaves are pinnately lobed.
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Common Tansy is native to the temperate portions of Eurasia and has been introduced into the North America for its medicinal and ornamental properties (Jacobs 2008; Mosley 2013; LeCain and Sheley 2014). Its native habitat is the sub-alpine mountain river valleys in Siberia. Most occurrences in Europe are associated with humans, who brought it to North America in the 1600s (Jacobs 2008; LeCain and Sheley 2014). It was recommended as a necessary plant for colonial herb gardens where it was cultivated in New England in 1638 and 1663 (Jacobs 2008; LeCain and Sheley 2014). Since then it has spread from gardens and was considered naturalized through the northeast by 1785 (Jacobs 2008).
Common Tansy was first documented in Madison County, Montana in 1931 along a road near a sawmill at 7,500 feet elevation on the Gallatin National Forest (Consortium of Pacific Northwest Herbaria (CPNWH); http://www.pnwherbaria.org). Since then it has been found along streams and lakes, roads, railroad tracks, on rangeland, and in disturbed meadows, vacant lots, irrigated pastures, and others (CPNWH).
Adhikari et al. (2020) modeled the predicted future distribution of this and other weed species across the major road network in Montana under predicted future climate scenarios and found that area of predicted future habitat suitability is likely to decrease.
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
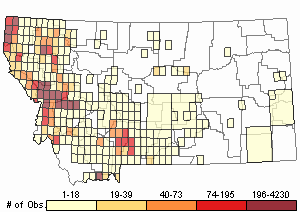
Observations in Montana Natural Heritage Program Database
Number of Observations: 23022
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
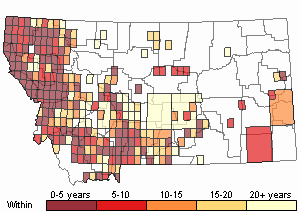
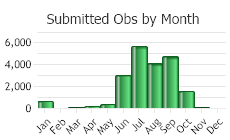
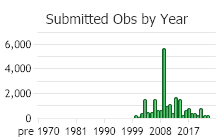
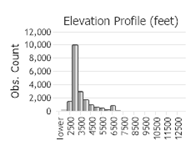 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
In Montana it grows in moist, disturbed meadows, often along streams or lakes, along roadsides and railroad tracks in the plains and valleys (Jacobs 2008; Lesica et al. 2012).
Ecology
Flies, butterflies, moths, and honeybees pollinate Common Tansy flowers (LeCain and Sheley 2017).
Potential and Known Pollinators:
The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus bifarius,
Bombus rufocinctus,
Bombus ternarius,
Bombus occidentalis, and
Bombus flavidus (Thorp et al. 1983, Colla and Dumesh 2010, Koch et al. 2012).
Common Tansy is a strong competitor in low productive soils because its root system can easily acquire nutrients (Jacobs 2008). It can often be found in near-monocultures on sandy soils in riparian areas (Jacobs 2008). On high productive soils, Common Tansy is very productive, but may be more susceptible to defoliation by slugs which could shift competition in favor of rhizomatous grasses and forbs (Jacobs 2008). Readers are advised to consult Rebele (2000) for the study that led to these conclusions.
CULTURALCommon Tansy has traditionally been considered to have insecticidal, antimicrobial, and medicinal properties (Duke 1987; Keskitoal 1999). It is reported as being used as a medicine since the times of the Ancient Greeks (LeCain and Sheley 2014). During the Middle Ages spanning the 5th to the 15th Century it is reported to be used for intestinal worms, rheumatism, fevers, digestive problems, and in large doses used to induce abortion (LeCain and Sheley 2014).
Common Tansy was used in early American history for preservation (LeCain and Sheley 2017). A governor for Massachusetts in the 1600s listed Common Tansy as a necessary plant for herb gardens (LeCain and Sheley 2014). Plants were used in burials and for packing meats and other perishables (LeCain and Sheley 2014).
The essential oils of Common Tansy have repellent properties. In the Middle Ages Common Tansy was used to repel mosquitos (LeCain and Sheley 2014). Essential oil from Common Tansy has been shown to repel the Colorado Potato Beetle (
Leptinotarsa decemlineata) (Panasiuk 1984; Schearer 1984). Interplanting Common Tansy and potato plants reduced beetle populations by 60-100% (Panasiuk 1984; Schearer 1984). Another study found that particular constituents (alpha-terpineol, 4-terpineol, and alpha- plus beta-thujone, 1,8-cineole, verbenol, and verbenone) when tested separately were able to repel ticks in the laboratory (Palsson et al. 2008). The authors suggest these substances are candidates in creating an effective repellent blend, and that field studies were forthcoming (Palsson et al. 2008).
From European populations of Common Tansy, about 57 compounds have been identified and categorized into 4 main groups: 1,8-cineole, trans-thujone, camphor, and myrtenol (Jacobs 2008; Judzentiene and Mockute 2005). The amount and ratios of oil groups produced by a plant will depend upon its population, site and environmental conditions, and time of year.
* 1,8-cineolis is a monoterpene cyclic ether, used by Common Tansy as a toxin to defend itself (its leaves) against herbivory. Biological activities of 1,8-cineolis includes: allelopathy, anesthetic, antibacterial, carcinogenic, fungicide, herbicide, insectifuge, nematicide, sedative, and testosterone hydroxylase iducer.
* Thugone is a monoterpene with a menthol-like aroma. In the 19th Century it was mixed in liquor (absinthe) and consumed to increase brain activity, develop new ideas, expand imagination, cause hallucinations, and as an aphrodisiac. These effects haven been disproven, and shown that it is a GABA receptor antagonist that allows neurons to fire more easily and cause spasms. Van Gogh consumed such liquor and is reported to have experienced fits of convulsions.
* Camphor is a terpene. It is used in the manufacturing of plastics, in lacquers, varnishes, explosives, and pyrotechnics, as a moth repellent, and as a preservative in pharmaceuticals and cosmetics. It is readily absorbed by the skin and can relieve itches and pain and creates a sense of coolness. It is also used as an antibacterial injection for infected root canals.
* Myrtenol is an oxygenated monoterpene. It attracts pine bark beetles and is used to bait them. It has also been used as a beverage preservative, a flavoring, and a fragrance.
Reproductive Characteristics
Plants reproduce from seeds and rhizomes.
RHIZOMES
Rhizomes mainly grow within the upper two feet of the soil horizon, which is relatively shallow soil. They are coarse, wiry, and have minimal development of fine hairs. These characteristics suggest that are competitive and efficient in taking up nutrients and water. Buds on the rhizomes can grow into stems or another rhizome.
FRUITS
Fruits are achenes. Each achene about 1–2 mm long, cylindric, 4-5 angled, and containing 1 seed (Lesica et al. 2012). A Montana study in Gallatin County estimated 9,966 flowers per plant (Jacobs 2008). They observed that many achenes were not developed and estimated 2,553 achenes per plant and 198,625 achenes per square meter (Jacobs 2008). About 75% of seeds collected in October germinated after one month of cold stratification implying that seeds have high viability and can germinate upon reaching maturity.
LIFE CYCLE [Adapted from Jacobs 2008]
This is based on Montana observations: In Montana stems can develop from rhizome buds in November; however, Stems typically emerge in spring after many perennial grasses have emerged. Leaves grow from mid-May to Mid-June and flower buds form in June. Plants bloom from June to August. Stems and leaves die as soil moisture depletes. Flowering and/or seed production is presumed to occur into October and November.
Seeds remain within drying flower heads unless physically dislodged. The leverage action caused by the drying of tall, stiff stems disperses seeds at or near to the parent plants. Seeds can be dispersed by wind or on water currents. Seeds in muddy soil can be transported by the foot action of humans and other animals. It is unknown if birds eat seeds or disperse them. Plants also spread by their rhizomes.
Management
An integrated vegetative management approach provides the best long-term control, and requires that land-use objectives and a desired plant community be identified (Shelly et al.
in Sheley and Petroff 1999). Once identified then an integrated weed management strategy that promotes a weed-resistant plant community and serves other land-use objectives such as livestock forage, wildlife habitat, or recreation can be developed, making control of spotted knapweed possible (Shelly et al.
in Sheley and Petroff 1999).
PREVENTION [Adapted from Jacobs 2008]
Seed development must be prevented to reduce or stop spread.
* Prevent vehicles from driving through and animals from grazing within infested areas,
* Thoroughly wash the undercarriage of vehicles and wheels in a designated area before moving to an uninfested area,
* Encourage landowners to frequently monitor their land for new infestations and, when found to implement effective control methods.
* Maintain proper livestock grazing management that is more resilient to Common Tansy invasion, and
* Develop educational campaigns to teach people to not pick and transport the yellow flowers.
PHYSICAL and CULTURAL CONTROLS [Adapted from Jacobs 2008]
Hand-pulling is effective for small populations. Hand-pulling should be done when soils are moist in order to remove all rhizomes. Rhizomes can re-sprout. Follow-up treatments will be necessary until all rhizomes and the seed bank are deleted.
Mowing reduces flower and seed production if done before the bloom stage. To maintain the vigor of desirable plants it is recommended to keep a 4-inch or greater stubble height. Plants in seed should not be mowed because it will spread them.
Mowers must be carefully and thoroughly cleaned to prevent spreading seeds.
Tilling can control Common Tansy where it occurs in fields. However, disturbing the soil can cause germination and/or fragmented rhizomes to grow. Follow-up treatments will be necessary until all rhizomes and the seed bank are deleted.
Farming equipment must be carefully and thoroughly cleaned to prevent spreading seeds.
Prescribed burning should be integrated with other control methods, such as herbicide, grazing, and revegetation management. Burned plants can regenerate from rhizomes. Burning can remove dense dried-up litter. Burning can be used on actively growing plants to remove dead material in preparation for an herbicide application.
Revegetation should be integrated with other control methods, such as herbicide or hand-pulling. Plant competition reduces the invasiveness of Common Tansy and increases the effectiveness of control applications. Suppressing the population through hand-pulling or an herbicide application will help in re-vegetation efforts. Species selected for re-vegetating should be appropriate for management objectives, adapted to the site conditions, and be competitive. Planting with appropriate native plants is highly encouraged. Refer to Montana Plant Materials Technical Note 46,
Seeding Rates for Conservation Species for Montana, and Extension Bulletin EB0019,
Dryland Pasture Species for Montana and Wyoming for possible species selection and seeding rates. Common tansy is often found in all the hydrologic zones of stream banks and riparian areas. Conservation practices that address riparian restoration, such as Channel Bank Vegetation (Code 322), may be needed after common tansy control to maintain hydrological cycles and prevent soil and water resource concerns such as erosion and sedimentation.
GRAZING CONTROLS [Adapted from Jacobs 2008]
Common Tansy is reported to be toxic to livestock. Cattle in the mid-west have reported to abort their fetus. Some ingestion might be okay and wild ungulates might be more tolerant to the plant. It has been suggested that herbivores have a toxin blood-level feedback mechanism, whereby, that when a certain threshold is reached animals will no longer graze Common Tansy.
Sheep have been used to manage Common Tansy in Montana. However, it is recommended that that female sheep be removed from areas with Common Tansy four weeks prior to breeding to avoid any potential reproductive problems. In a Montana study, sheep reduced the above-ground biomass of Common Tansy by 90% while consuming similar levels of perennial grasses. Long-term effects from grazing are unknown, but it is hypothesized that Common Tansy populations would decline allowing perennial grasses to increase.
CHEMICAL CONTROLS [Adapted from Jacobs 2008]
An integrated vegetative management approach provides the best long-term control, and requires that land-use objectives and a desired plant community be identified (Shelly et al.
in Sheley and Petroff 1999). Once identified then an integrated weed management strategy that promotes a weed-resistant plant community and serves other land-use objectives such as livestock forage, wildlife habitat, or recreation can be developed, making control of spotted knapweed possible (Shelly et al.
in Sheley and Petroff 1999). For up to date information on herbicides, consult
Greenbook.
Common Tansy had been controlled using Metsulfuron, chlorsulfuron, or by mixing them. These chemicals are not selective and can kill shrubs, grasses, and other broad-leaf plants. Applications can be applied to plants growing to the water’s edge, but must
not be applied directly to surface water of any depth. A non-ionic surfactant at 0.5% volume/volume or methylated seed oil at 2% volume/volume in a spray solution is recommended. Visible effects of the herbicides may not be apparent until 45 days after the treatment.
*
metsulfuron applied at a broadcast rate of 0.5 ounces per acre at the late bud stage (late June) provided almost 100% control for one year for a population growing on moist soil.
*
metsulfuron and
chlorosulfuron mixed together at the individual rate of 0.25 ounce per acre had similar results as above.
At sites without a high water table,
Picloram applied to actively growing plants in the bud to bloom stages can be used to control Common Tansy.
Picloram mixed with
dicamba and applied to the budg stage provided 98% control for 24 months after treatment.
Where Common Tansy is growing in water,
imazapyr applied at 1 quart per acre may provide some control.
Useful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesStewardship Responsibility
Threats or Limiting Factors
Common Tansy reduces available forage because cattle and horses generally avoid them (Jacobs 2008). The volatile oils in the leaves deter grazing by livestock, and native ungulates are rarely observed eating them.
Common Tansy can form dense patches which out-compete native plants and reduces bio-diversity (Jacobs 2008).
It may reduce habitat for pollinating insects (Jacobs 2008).
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Adhikari, A., L.J. Rew, K.P. Mainali, S. Adhikari, and B.D. Maxwell. 2020. Future distribution of invasive weed species across the major road network in the state of Montana, USA. Regional Environmental Change 20(60):1-14. https://doi.org/10.1007/s10113-020-01647-0
Adhikari, A., L.J. Rew, K.P. Mainali, S. Adhikari, and B.D. Maxwell. 2020. Future distribution of invasive weed species across the major road network in the state of Montana, USA. Regional Environmental Change 20(60):1-14. https://doi.org/10.1007/s10113-020-01647-0 Duke, J.A. 1987. CRC handbook of medicinal plants. CRC, Boca Raton, Florida.
Duke, J.A. 1987. CRC handbook of medicinal plants. CRC, Boca Raton, Florida. Flora of North America Editorial Committee. 2006. Flora of North America North of Mexico. Vol. 19. Magnoliophyta: Asteridae, part 6: Asteraceae, part 1. Oxford Univ. Press, New York. xxiv + 579 pp.
Flora of North America Editorial Committee. 2006. Flora of North America North of Mexico. Vol. 19. Magnoliophyta: Asteridae, part 6: Asteraceae, part 1. Oxford Univ. Press, New York. xxiv + 579 pp. Jacobs, Jim. 2008. Ecology and Management of Common Tansy (Tanacetum vulgare L.)
Jacobs, Jim. 2008. Ecology and Management of Common Tansy (Tanacetum vulgare L.) Judzentiene, A. and D. Mockute. 2005. The inflorescence and leaf essential oils of Tanacetum vulgare L. var. vulgare growing wild in Lithuania. Biochemical Systematics and Ecology 33: 487-498.
Judzentiene, A. and D. Mockute. 2005. The inflorescence and leaf essential oils of Tanacetum vulgare L. var. vulgare growing wild in Lithuania. Biochemical Systematics and Ecology 33: 487-498. Keskitalo, M.K. 1999. Exploring biodiversity to enhance bioactivity in the genus Tanacetum through protoplast fusion. Academic dissertation, University of Finland, Department of Plant Production, Section of Crop Husbandry, Publication Number 53.
Keskitalo, M.K. 1999. Exploring biodiversity to enhance bioactivity in the genus Tanacetum through protoplast fusion. Academic dissertation, University of Finland, Department of Plant Production, Section of Crop Husbandry, Publication Number 53. LeCain, Ron and Roger Sheley. 2014. Common tansy (Tanacetum vulgare). MontGuide MT199911AG. Revised January 2014. Montana State University-Extension, Bozeman, Montana.
LeCain, Ron and Roger Sheley. 2014. Common tansy (Tanacetum vulgare). MontGuide MT199911AG. Revised January 2014. Montana State University-Extension, Bozeman, Montana. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Palsson, Katinka, Thomas G.T. Jaenson, Peter Baeckstrom, and Anna-Karin Borg-Karlson. 2008. Tick Repellent Substances in the Essential Oil of Tanacetum vulgare. Journal of Medical Entomology. 45(10: 88-93.
Palsson, Katinka, Thomas G.T. Jaenson, Peter Baeckstrom, and Anna-Karin Borg-Karlson. 2008. Tick Repellent Substances in the Essential Oil of Tanacetum vulgare. Journal of Medical Entomology. 45(10: 88-93. Robele, F. 2000. Competition and coexistence of rhizomatous perennial plants along a nutrient gradient. Plant Ecology 147: 77-94.
Robele, F. 2000. Competition and coexistence of rhizomatous perennial plants along a nutrient gradient. Plant Ecology 147: 77-94. Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Boggs, K. W. 1984. Succession in riparian communities of the lower Yellowstone River, Montana. M.S. Thesis. Montana State University, Bozeman, 107 pp.
Boggs, K. W. 1984. Succession in riparian communities of the lower Yellowstone River, Montana. M.S. Thesis. Montana State University, Bozeman, 107 pp. Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp.
Culver, D.R. 1994. Floristic analysis of the Centennial Region, Montana. M.Sc. Thesis. Montana State University, Bozeman. 199 pp. Delphia, C.M., Griswold, T., Reese, E.G., O'Neill, K.M., and Burkle, L.A. 2019. Checklist of bees (Hymenoptera: Apoidea) from small, diversified vegetable farms in south-western Montana. Biodiversity Data Journal: e30062
Delphia, C.M., Griswold, T., Reese, E.G., O'Neill, K.M., and Burkle, L.A. 2019. Checklist of bees (Hymenoptera: Apoidea) from small, diversified vegetable farms in south-western Montana. Biodiversity Data Journal: e30062 Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p.
Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p. Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p.
Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p. Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p.
Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p. Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park.
Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park.
- Web Search Engines for Articles on "Common Tansy"





