View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Northern Leopard Frog - Lithobates pipiens
State Rank Reason (see State Rank above)
Species has suffered declines west of the continental divide possibly due to the introduction of Chytrid Fungus. Impacts to the species in eastern and central Montana were negligible where the species remains common. Reintroduction efforts in the Flathead Valley have successfully established a viable population and recovery in this area appears possible. Threats to the species include habitat loss and degradation due to drought and pollution from agricultural runoff. It is unknown if future climate changes will exacerbate impacts of Chytrid. As eastern populations are doing well, the calculated status rank does not account for the near extirpation of western populations. As such, the Montana Species of Concern Committee voted to lower the rank to S3S4 to highlight the near loss of this population and potential for reintroduction of the species. The species should be considered a Species of Concern west of the Continental Divide, but common to the east.
General Description
EGGS:
Eggs are laid in a single orange to grapefruit-sized, somewhat flattened globular mass and are usually attached to submerged vegetation. Egg masses are laid individually or communally in groups of up to three dozen egg masses (Nussbaum et al. 1983). Egg masses contain from 645 to 6,272 eggs (X = 3,045, N = 68 for completely counted egg masses at five sites in Colorado and Wyoming) (Corn and Livo 1989). Each ovum is black above, white below, and is surrounded by two jelly layers (Livezey and Wright 1947). Ovum diameters are approximately 1.7 mm (0.07 in), but total egg diameters, including the jelly layers, are approximately 5.0 mm (0.2 in) (Livezey and Wright 1947).
LARVAE:
Body and tail musculature are dark brown to olive or gray with flecks of light gold or silver and black (Bryce Maxell, personal observation). The tail musculature may be pale (Corkran and Thoms 2006). The lateral body surface has a larger proportion of light gold or silver flecks, and the ventral body surface is silvery white to transparent. The tail fin is clear to yellowish with black and light gold or silver flecks and is less than 1.5 times the body length (Bryce Maxell, personal observation). The dorsal tail fin begins anterior to the tail musculature when viewed from the side. The anus is on the right side in front of the fin, not on the midline. The eyes fall within the outline of the head when viewed from above. Lateral oral papillae are strongly indented toward the corners of the mouth, and the lower mandible is noticeably thicker than the upper. (Werner et al. 2004). Tadpoles have a total length of 5.5-100 mm (0.22-3.9 in) (Livo 1981 as cited in Hammerson 1999, Hammerson 1999).
JUVENILES AND ADULTS:
White to cream stripes extend from the tip of the snout laterally underneath the eye to just above the base of the front limb and from just behind each eye to the base of the hind limbs (Bryce Maxell, personal observation). Dorsal base color is either green or brown with large, oval shaped, black spots that are regular in outline and are surrounded with a light halo (Fogleman et al. 1980). Individuals occasionally have a blue to light blue base color (Black 1969a, Hammerson 1999). Ventral color is white to cream with some pinkish patches, especially on the feet. The hind feet have extensive webbing (Bryce Maxell, personal observation) with a snout-vent Length (SVL) of 18-110 mm (0.7-4.3 in) (Nussbaum et al. 1983, Hammerson 1999).
VOICE:
Although both male and female frogs can make croaks, males are typically louder. The mating calls are short (2-3 seconds) and grating sequence of notes with chortles followed by guttural clucking, grunts and squeaks (Werner et al. 2004). Calls are not very loud and can be heard up to a distance of 20 m (65.6 ft) (Bryce Maxell, personal communication).
Diagnostic Characteristics
Adult Columbia Spotted Frogs (
Rana luteiventris) often have red or salmon color on their ventral surface. Their dorsal surface has small, irregularly shaped black spots with white or light-yellow centers. Adult Bullfrogs (
Lithobates catesbeianus) lack the white to yellowish stripe on the lateral portion of the snout and have tympanums that are the same size or larger than their eye. There is a fold of skin that extends from the back of their eye, over their tympanum, down to their front leg.
Larval Columbia Spotted Frogs have tails that are usually twice their body length with large flecks of black on their body or tail. They often have a metallic copper sheen on the lateral edges of their ventral surfaces. Larvae of the American Bullfrog have a bright to creamy yellow ventral surface with perfectly round black dots on their dorsal surface and tail musculature. The tadpole of this species is much larger in size.
The eggs of Columbia Spotted Frog have diameters approximately twice those of the Northern Leopard Frog because their jelly envelopes are much larger (see descriptions). Additionally, the egg masses of the Columbia Spotted Frog are usually at the water’s surface and not attached to vegetation (Ross et al. 1994b). American Bullfrog eggs are laid in the middle of the summer and are spread out in a thin layer over the surface or bottom of a pond rather than a globular mass.
In Montana, extant populations of Northern Leopard Frog overlap Columbia Spotted Frog and American Bullfrog in very few locations. Northern Leopard Frogs are present mostly across the prairies of the eastern two-thirds of the state while the Columbia Spotted Frog and most American Bullfrog populations are in the mountainous western third. See species accounts for distribution to identify possible regions of co-occurrence.
Species Range
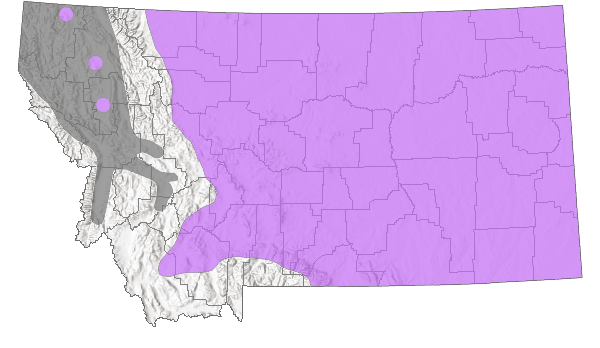
Montana Range
Range Descriptions
 Global Range
Global Range

Estimated global breeding range area is: 5,617,102 km
2 (Approximately 5% in Montana)
See information about
 iNaturalist Geomodels
iNaturalist Geomodels
Range Comments
The Northern Leopard Frog has a complex taxonomic history but is now recognized as a distinct species that historically ranged from Newfoundland and northern Alberta in the north to the Great Lakes region, the desert Southwest, and the Great Basin in the south (Pace 1974, Dunlap and Platz 1981, Hillis 1988). In addition, a number of isolated populations historically existed in the Pacific Northwest and California (Stebbins 2003). Across this range populations have been documented at elevations up to 3,350 m (11,000 ft) (Hammerson 1999).
In Montana they have historically been documented across the eastern plains and in many of the mountain valleys on both sides of the Continental Divide. Unfortunately, over the last few decades populations of the Northern Leopard Frog have undergone declines and extinctions across much of the western portion of their range (Stebbins and Cohen 1995). Most Northern Leopard Frog populations in western Montana apparently became extinct sometime in the late 1970’s or early 1980’s when virtually no amphibian studies were being conducted in the state. Only two population centers are now known to exist in western Montana, one near Kalispell and one near Eureka (Werner et al. 1998a, Kirwin Werner, Salish Kootenai College, personal communication). In addition, out of 47 historic sites revisited in the mid-1990’s in central Montana, Northern Leopard Frogs were only found at 9 (19%) (Reichel 1995a, 1996, Koch et al. 1996). Populations in southeastern Montana still seem to be widespread and abundant (Reichel 1995b, Hendricks and Reichel 1996b, Koch et al. 1996).
Maximum elevation: 2,042 m (6,700 ft) in Judith Basin County (Maxell et al. 2003).
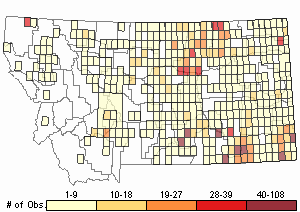
Observations in Montana Natural Heritage Program Database
Number of Observations: 2931
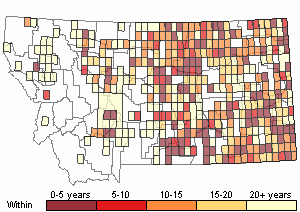
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
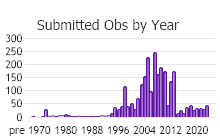
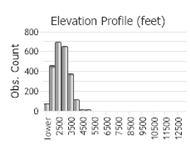 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Migration
No information on migration of the Northern Leopard Frog is available for Montana. In other locations, Northern Leopard Frogs usually remain in relatively small seasonal home ranges, but may range several hundred meters or more between seasons in the upper Midwest. In Michigan, average nightly movement during rain was 36 m (118 ft), and as much as 800 m (2625 ft). Individuals in Colorado have been documented moving at least 3 km (1.9 mi) between years, and 8 km (5 mi) between-year movements have been reported in the Cypress Hills, Alberta; young-of-the-year moved 2.1 km (1.3 mi) between natal and breeding ponds in the Cypress Hills (Wagner 1997, Hammerson 1999).
Habitat
In Montana, the Northern Leopard Frog is typically found in and adjacent to low elevation and valley bottom ponds, spillway ponds, beaver ponds, stock reservoirs, lakes, creeks, pools in intermittent streams, warm water springs, potholes, and marshes (Brunson and Demaree 1951, Mosimann and Rabb 1952, Black 1969a, Miller 1978, Dood 1980, Reichel 1995a, 1995b Hendricks and Reichel 1996a, 1996b, Hendricks 1999a). Habitats tend to be permanent slow moving or standing water bodies with considerable rooted aquatic vegetation. However, individuals may range widely into moist meadows, grassy woodlands, and even agricultural areas (Nussbaum et al. 1983). In Montana adults are found primarily in riparian habitats or on the prairies near permanent waters without tall dense vegetation (Mosimann and Rabb 1952, Black 1969a, Miller 1978). There is no evidence that this species in Montana has ever occupied high elevation wetlands, in contrast to Wyoming and Colorado (Baxter and Stone 1985, Hammerson 1999).
Northern Leopard Frogs require a mosaic of habitats to meet annual requirements of all life stages. Generally separate sites are used for breeding and overwintering, but this may occur in the same pond in some cases. In summer, adults and juveniles commonly feed in open or semi-open wet meadows and fields with shorter vegetation, usually near the margins of water bodies, and seek cover underwater; taller, denser vegetation seems to be avoided. Adults overwinter on the bottom surface of permanent water bodies, under rubble in streams, or in underground crevices that do not freeze and are well oxygenated (Rand 1950, Emery et al. 1972, Baxter and Stone 1985, Cunjak 1986, Russell and Bauer 1993, Wagner 1997, Hammerson 1999).
National Vegetation Classification System Groups Associated with this Species
Forest and Woodland
Deciduous Forest and Woodland
Wetland and Riparian
Alkaline - Saline Wetlands
Alpine Riparian and Wetland
Peatland
Riparian and Wetland Forest
Riparian Shrubland
Wet Meadow and Marsh
Recently Disturbed or Modified
Introduced Vegetation
Human Land Use
Agriculture
Food Habits
Adults feed on a variety of small invertebrates, including various insects, spiders, leeches, and snails obtained along the water's edge or in nearby meadows or fields. but may cannibalize smaller individuals and ingest plant matter incidentally (Knowlton 1944, Moore and Strickland 1954, Whitaker 1961, Linzey 1967, Miller 1975, 1978 Nussbaum et al. 1983, Russell and Bauer 1993, Wagner 1997,). Tadpoles feed on a variety of algae as well as detritus (DeBenedictis 1974). In Montana, adults have been documented feeding on 10 orders of insects, spiders, mites, harvestmen, centipedes, millipedes, snails, and newly metamorphosed Western Toads (
Anaxyrus boreas) (Miller 1978), but larval food habits have not been described.
Ecology
Northern Leopard Frogs are active during the day and night. The active period extends from March to November in Colorado (Hammerson 1999). In Wyoming and the Pacific Northwest, adults emerge in March or April (Nussbaum et al. 1983, Baxter and Stone 1985, Russell and Bauer 1993) when water temperatures exceed 10 °C (50 °F). In Montana, the active period of adults is reported to extend from mid-March to early October (Brunson and Demaree 1951, Roedel and Hendricks 1998a, 1998b, Hendricks 1999a). In all cases, activity begins when ice melts.
Adults typically do not move more than 50 m (164 ft) within a seasonal home range but may migrate several hundred to a thousand or more meters between seasonal home ranges (Dumas 1964, Dole 1965a, 1965b, Dole 1967a, 1967b, Dole 1968). Juveniles are known to disperse up to 8.0 km (5 mi) from their natal ponds to their adult seasonal territories (Dole 1971, Seburn et al. 1997).
Predators of adults and juveniles include Great Blue Heron (
Ardea herodias), Burrowing Owl (
Athene cunicularia), snakes (including gartersnakes), some mammalian carnivores, and game fish. Tadpole predators include Pied-billed Grebe (
Podilymbus podiceps), Western Tiger Salamander (
Ambystoma mavortium), gartersnakes, and American Bullfrog (
Lithobates catesbeianus) tadpoles (Nussbaum et al. 1983, Russell and Bauer 1993, Hammerson 1999). Predators in Montana have not been reported.
Northern Leopard Frogs apparently out-competed Columbia Spotted Frogs at low elevations in Montana (Black 1969a). Differential tadpole mortality may be the primary mechanism of displacement of Columbia Spotted Frog by Northern Leopard Frog (Dumas 1966).
Reproductive Characteristics
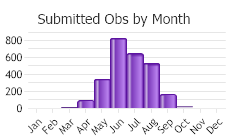
Information on reproduction in Montana is limited, and no detailed studies of the reproductive biology of any population have been conducted. Timing appears variable and depends on the year and location. Calling males have been reported in April and May. Near Tiber Reservoir, in Toole and Liberty counties, females have been collected with relatively undeveloped eggs in mid-June and moderately developed to fully developed eggs in early and late July; recently transformed juveniles were also noted in late July (Mosimann and Rabb 1952). Eggs and tadpoles have been reported at breeding sites across eastern Montana during early April to late July, with a peak in May and June; sometimes tadpoles are observed in August and September (Reichel 1995b, Hendricks and Reichel 1996b, Hendricks 1999a, Hossack et al. 2003). Recently metamorphosed juveniles with small tail stubs measured 26-34 mm (1-1.3 in) SVL.
In general, males gather at breeding sites of shallow, quiet water in March to April and vocalize on warm sunny days (water temperatures of 14 to 23 °C (57-73 °F) while floating at the surface of the water. The breeding call of males is a snoring sound lasting 2 to 3 seconds followed by a series of 2 to 3 stuttering croaks or chuckles. In favorable habitat, 20 to 25 or more males may gather in a 20 square meter (65.6 square ft) area.
Females begin laying eggs a few days after calling starts. The time of egg deposition varies with latitude and elevation. Egg deposition occurs typically in April in southern Quebec, New York, and the Great Lakes region, late April to late May farther north in Manitoba and Nova Scotia (Gilbert et al. 1994). In Colorado, eggs are laid mainly in late March or by mid-April at low elevations, and in May in the mountains (Corn and Livo 1989, Hammerson 1999). Females will deposit egg masses singly or communally in waters 7-25 cm (2.8-9.8 in) deep attached to vegetation under the water’s surface in areas well exposed to sunlight (Corn and Livo 1989, Werner and Reichel 1996, Hammerson 1999). Breeding often peaks when water temperatures reach about 10 °C (50 °F). At a particular site, egg deposition generally occurs within a span of about 10 days. Egg masses include several hundred to several thousand ova; the clutch size of 68 Colorado egg masses were 645 to 6272 eggs (Corn and Livo 1989). The density of egg masses often reaches a few hundred per hectare in favorable habitat, sometimes more than 1000 per hectare, but is usually less than 100 in Colorado.
Eggs hatch in about 1 to 2 weeks with hatching taking place over several weeks at a single site. The larval period is about 10 to 12 weeks (58 to 105 days) with recently metamorphosed juveniles documented in late June and early July at lower elevations, and in mid-July to September at higher elevations (Corn 1981, Hammerson 1999). Size at metamorphosis is 21-36 mm (0.8-1.4 in) SVL. Aquatic larvae usually metamorphose in summer, but they may overwinter as tadpoles in some areas (Baxter and Stone 1985). Females are sexually mature usually in two years in most areas, three years in high elevation populations. Breeding males in Colorado are usually more than 5.0 cm (2.0 in) SVL, and breeding females more than 6.0 cm (2.4 in). In northern Minnesota, successful reproduction in acidic bog water either does not occur or is a rare event (Karns 1992).
Management
The following was taken from the Status and Conservation section for the Northern Leopard Frog account in
Maxell et al. 2009Within the last twenty to twenty-five years Northern Leopard Frog populations have declined and been extirpated from large portions of the area from the western plains of Colorado, Wyoming, Montana, and Alberta westward to Oregon and Washington (Roberts 1981, 1987, 1992, Corn and Fogleman 1984, Baxter and Stone 1985, Stebbins and Cohen 1995, Koch et al. 1996, Leonard and McAllister 1996, Leonard et al. 1999, Hammerson 1999). Suggested causes of declines in Northern Leopard Frog populations in this and other areas of the country included loss of wetlands and natural hydrological regimes, introductions of game fish, mosquitofish, and American Bullfrogs, application of pesticides and herbicides, and drought (Roberts 1981, 1987, 1992, Corn and Fogleman 1984, Koch and Peterson 1995, Stebbins and Cohen 1995, Leonard and McAllister 1996, Leonard et al. 1999, Hammerson 1999). While it is likely that all of these factors have played a role in the decline and extirpation of local Northern Leopard Frog populations, many of the declines and extirpations were apparently associated with regional mass mortality events between 1973 and 1982 because declines were observed in relatively pristine areas as well (Roberts 1981, 1987, 1991, 1992, Corn and Fogleman 1984, Koch and Peterson 1995). Reintroduction programs have been initiated in Alberta (Roberts 1991) and have been called for in Washington state (Leonard et al. 1999). The same general timeline for declines is evident in western Montana. Northern Leopard Frog populations were encountered and found to be apparently healthy by a number of masters and doctoral degree students between 1967 and 1977 (Black 1967b, 1970a, Miller 1975, Anderson 1977, Daugherty et al. 1978). A student at the Salish-Kootenai College noted that while he found Northern Leopard Frogs near Kicking Horse Reservoir on the Flathead Indian Reservation during the summer of 1980, local fisherman reported that they had noticed a definite decrease in the number of Northern Leopard Frogs in the area (Ryan 1980). Very little, if any, work was conducted on amphibians in Montana in the 1980s and surveys in the 1990s failed to find Northern Leopard Frogs at any of the historical sites that were revisited and only found two remaining populations in all of western Montana west of the Continental Divide (Werner and Plummer 1995a, 1995b, Werner and Reichel 1994, 1996, Hendricks and Reichel 1996a, Koch et al. 1996, Werner et al. 1998a). Furthermore, while surveys during the 1990’s found them to be common east of the island mountain ranges in eastern Montana, they appeared to have been extirpated from 80% of historic localities on the northwestern plains (Reichel 1995a, 1995b, Hendricks and Reichel 1996a, 1996b Koch et al. 1996, Reichel 1996b, Reichel 1997, Hendricks and Reichel 1998, Rauscher 1998, Roedel and Hendricks 1998a, 1998b, Hendricks 1999a). As a result of these findings the USFS listed the Northern Leopard Frog as a sensitive species in all Region 1 Forests (USDAFS 1999). Risk factors relevant to the viability of populations of this species are likely to include all the risk factors described above. Individual studies that specifically identify risk factors or other issues relevant to the conservation of Northern Leopard Frogs include the following. (1) In conjunction with similar observations for Western Toads (
Anaxyrus boreas) Carey (1993) observed the disappearance of two populations of Northern Leopard Frogs in the West Elk Mountains of Colorado between 1974 and 1982. During this period, she found Northern Leopard Frogs with symptoms of red-leg disease, a common bacterial infection in amphibians and fish. She hypothesized that an unidentified environmental factor had caused sublethal stress of both species, which caused immune response to be suppressed, leading to the systemic infection and death. More recently the Amphibian Chytrid Fungus (
Batrachochytrium dendrobatidis ), which is suspected to be responsible for declines of amphibians in Australia, Central America, and the western United States, has been found to have caused mass mortalities in Northern Leopard Frog populations in southern Arizona during the summer of 1999 (Berger et al. 1998, Daszak et al. 1999, 2000, Morell 1999, Milius 1999). As was observed for declines in the late 1970’s and early 1980’s only metamorphosed individuals died (Morell 1999). The fungus only seems to attack keratinized tissues, so metamorphosed individuals with lots of keratinized tissues die and tadpoles with keratinized tissues only around the mouthparts survive until metamorphosis (Berger et al. 1998, Morell 1999). Furthermore, it now appears that the Amphibian Chytrid Fungus was responsible for declines in the late 1970’s and early 1980’s as well because museum specimens of Northern Leopard Frogs that were collected during these time period have now been found to have the Amphibian Chytrid Fungus (Carey et al. 1999, Daszak et al. 1999, Milius 2000). Thus, the Amphibian Chytrid Fungus may be the most likely cause of declines of Northern Leopard Frog populations in the western United States and in western Montana in the late 1970’s and early 1980’s and clearly represents a threat to populations today. In support of Carey’s (1993) immunosuppression hypothesis Maniero and Carey (1997) found that Northern Leopard Frogs exposed to low temperatures (5 °C or 41 °F) significantly reduced the animal’s immune response. Thus, Northern Leopard Frogs may be particularly susceptible to the Amphibian Chytrid Fungus or other pathogens when emerging in the early spring or in the late fall or winter or when faced with some other environmental stressor (Carey et al. 1999). (2) Berrill et al. (1993) found that the pyrethroid pesticides permethrin and fenvalerate did not cause significant mortality of embryos when they were exposed to commonly applied levels for 22 to 96 hours. However, tadpole growth and response to a potential predator was delayed following exposure. Berrill et al. (1994) found that the insecticide fenitrothion and the herbicides triclopyr and hexazinone had no effects on embryos, but the fenitrothion and triclopyr did kill or paralyze new hatchlings at concentrations of 2.4 to 4.8 ppm and 4.0 to 8.0 ppm, respectively. Berrill and Bertram (1997) found that northern leopard frog embryos exposed to 6 herbicides (hexazinone, triclopyr ester, triallate, trifluralin, glyphosate, and bromoxynil) and 3 insecticides (permethrin, fenvalerate, and fenitrothion) at levels that are commonly found in areas where they are used on forests or crops in Canada hatched successfully with no unusual mortality. However, when tadpoles were exposed to the same levels, they suffered partial paralysis and the authors note that they would be likely to suffer high rates of mortality. Kaplan and Overpeck (1964) and Kaplan and Glaczenski (1965) found that a variety of organophosphate and halogenated hydrocarbon pesticides caused both red and white blood cell counts to decline in adult Northern Leopard Frogs and chronic exposures to concentrations of 1 ppm caused death in some individuals. Dial and Dial (1987) found that the aquatic herbicides diquat and paraquat did not reduce embryo survival or change hatching time when applied at concentrations of 0.1 to 2.0 ppm. However, at the same concentrations young tadpoles suffered significant mortality from both chemicals and 15-day old tadpoles suffered significant mortality from paraquat. (3) Hecnar (1995) found that acute and chronic toxic effects of ammonium nitrate were observed in Northern Leopard Frog tadpoles at concentrations that are commonly exceeded in agricultural areas. Acute exposures to ammonium nitrate fertilizers at 20 mg/L for 96 hours resulted in 50 percent mortality and significant weight loss in those individuals that survived. Chronic exposures to 10 mg/L for 100 days resulted in significantly lower survivorship. Cameron (1940) found that well water containing 1 ppm flourine caused embryo development to slow and time to hatching to decrease. Lande and Guttman (1973) found that embryos were not affected by copper sulfate at concentrations up to 1.56 mg/liter of copper, but the LD50 for tadpoles was 0.15 mg/liter and tadpole growth rates were decreased at concentrations of 0.06. (4) Hamilton (1941) found that rotenone applied at 0.1 mg/L caused mortality in larval through metamorphic life history stages of Northern Leopard Frogs over an 8- to 24-hour time period, respectively. Furthermore, Burress (1982) found that Pro-Noxfish applied at 5 µL/L caused substantial mortality in Northern Leopard Frogs. (5) Black (1969a) felt that exotic American Bullfrogs introduced in the Bitterroot Valley had led to declines in Northern Leopard Frog populations in the area. Similarly, Hammerson (1982) documented a decline in the abundance of Northern Leopard Frogs as American Bullfrog numbers increased at a site in Colorado. (6) Vatnick et al. (1999) found that adult Northern Leopard Frogs preferred a neutral pH in a choice test and found that when they were exposed to water of pH 5.5 for 10 days, they suffered 72% mortality while those exposed to a pH of 7.0 suffered only 3.5% mortality. Furthermore, frogs appeared to be much more sensitive to low pH immediately after emergence from hibernation. Those exposed to a pH of 5.5 immediately after emerging all died within 4 days while frogs exposed after they had completed breeding activities only suffered 58% mortality over a 10-day period. Freda et al. (1991) report that a pH below 4.6 causes mortality of embryos to increase significantly from controls and all embryos die when exposed to a pH of 4.2-4.5. Corn and Vertucci (1992) report an LC50 of embryos at a pH of 4.5. Freda and Dunson (1985) found that tadpoles raised at a pH of 4.4 grew slower than siblings raised at a pH of 5.8. Furthermore, older tadpoles had higher survival rates at low pH than younger tadpoles. Schlicter (1981) found that sperm motility decreased below pH 6.5 and no embryos survived below a pH of 4.8. Long et al. (1995) found that low pH and UV-B acted synergistically to cause mortality in Northern Leopard Frog embryos. Freda et al. (1990) found that at a pH below 4.8, aluminum complexed with dissolved organic carbon and became toxic to tadpoles. (7) Nash et al. (1970) found that loud noises resulted in an immobility reaction in Northern Leopard Frogs. This could leave them at greater risk of mortality from traffic or heavy machinery. (8) Ankley et al. (2000) found that limb deformities were more prevalent when tadpoles were exposed to higher levels of UV-B radiation.
Stewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication [USDAFS] USDA Forest Service. 1999. Update of U.S. Forest Service Northern Region Sensitive Species List. 12 March, 1999. Region 1 U.S. Forest Service Supervisors Office, Missoula, Mt. 20 P.
[USDAFS] USDA Forest Service. 1999. Update of U.S. Forest Service Northern Region Sensitive Species List. 12 March, 1999. Region 1 U.S. Forest Service Supervisors Office, Missoula, Mt. 20 P. Anderson, M.E. 1977. Aspects of the ecology of two sympatric species of Thamnophis and heavy metal accumulation with the species. M.S. thesis, University of Montana, Missoula. 147 pp.
Anderson, M.E. 1977. Aspects of the ecology of two sympatric species of Thamnophis and heavy metal accumulation with the species. M.S. thesis, University of Montana, Missoula. 147 pp. Ankley, G.T., J.E. Tietge, G.W. Holcombe, D.L. DeFoe, S.A. Diamond, K.M. Jensen, and S.J. Degitz. 2000. Effects of laboratory ultraviolet radaition and natural sunlight on survival and development of Rana pipiens. Canadian Journal of Zoology 78: 1092-1
Ankley, G.T., J.E. Tietge, G.W. Holcombe, D.L. DeFoe, S.A. Diamond, K.M. Jensen, and S.J. Degitz. 2000. Effects of laboratory ultraviolet radaition and natural sunlight on survival and development of Rana pipiens. Canadian Journal of Zoology 78: 1092-1 Baxter, G.T. and M.D. Stone. 1985. Amphibians and reptiles of Wyoming. Second edition. Wyoming Game and Fish Department. Cheyenne, WY. 137 p.
Baxter, G.T. and M.D. Stone. 1985. Amphibians and reptiles of Wyoming. Second edition. Wyoming Game and Fish Department. Cheyenne, WY. 137 p. Berger, L., R. Speare, P. Daszak, D.E. Green, A.A. Cunningham, C.L. Goggin, R. Slocombe, M.A. Ragan, A.D. Hyatt, K.R. McDonald, H.B. Hines, K.R. Lips, G. Marantelli and H. Parkes. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences 95: 9031-9036.
Berger, L., R. Speare, P. Daszak, D.E. Green, A.A. Cunningham, C.L. Goggin, R. Slocombe, M.A. Ragan, A.D. Hyatt, K.R. McDonald, H.B. Hines, K.R. Lips, G. Marantelli and H. Parkes. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proceedings of the National Academy of Sciences 95: 9031-9036. Berrill, M. and S. Bertram. 1997. Effects of pesticides on amphibian embryos and larvae. In: D.M. Green (ed.) Amphibians in decline: Canadian studies of a global problem. Society for the Study of Amphibians and Reptiles, Herpetological Conservation 1: 233-245.
Berrill, M. and S. Bertram. 1997. Effects of pesticides on amphibian embryos and larvae. In: D.M. Green (ed.) Amphibians in decline: Canadian studies of a global problem. Society for the Study of Amphibians and Reptiles, Herpetological Conservation 1: 233-245. Berrill, M., S. Bertram, A. Wilson, S. Louis, D. Brigham, and C. Stromberg. 1993. Lethan and sublethal impacts of pyrethroid insecticides on amphibian embryos and tadpoles. Environmental Toxicology and Chemistry 12:525-539.
Berrill, M., S. Bertram, A. Wilson, S. Louis, D. Brigham, and C. Stromberg. 1993. Lethan and sublethal impacts of pyrethroid insecticides on amphibian embryos and tadpoles. Environmental Toxicology and Chemistry 12:525-539. Berrill, M., S. Bertram, L. McGillivray, M. Kolohon, and B. Pauli. 1994. Effects of low concentrations of forest-use pesticides on frog embyos and tadpoles. Environmental Toxicology and Chemistry 13(4): 657-664.
Berrill, M., S. Bertram, L. McGillivray, M. Kolohon, and B. Pauli. 1994. Effects of low concentrations of forest-use pesticides on frog embyos and tadpoles. Environmental Toxicology and Chemistry 13(4): 657-664. Black, J.H. 1967b. A blue leopard frog from Montana. Herpetologica 23(4): 314-315.
Black, J.H. 1967b. A blue leopard frog from Montana. Herpetologica 23(4): 314-315. Black, J.H. 1969a. The frog genus Rana in Montana. Northwest Science 43(4): 191-195.
Black, J.H. 1969a. The frog genus Rana in Montana. Northwest Science 43(4): 191-195. Black, J.H. 1970a. Amphibians of Montana. Montana Wildlife, Montana Fish and Game Commission. Animals of Montana Series 1970(1): 1-32.
Black, J.H. 1970a. Amphibians of Montana. Montana Wildlife, Montana Fish and Game Commission. Animals of Montana Series 1970(1): 1-32. Brunson, R.B. and H.A. Demaree, Jr. 1951. The herpetology of the Mission Mountains, Montana. Copeia (4):306-308.
Brunson, R.B. and H.A. Demaree, Jr. 1951. The herpetology of the Mission Mountains, Montana. Copeia (4):306-308. Burress, R.M. 1982. Effects of synergized rotenone on nontarget organisms in ponds. In U.S. Fish and Wildlife Service, Washington DC, Investigations in Fish Control No. 91, pp. 1-7.
Burress, R.M. 1982. Effects of synergized rotenone on nontarget organisms in ponds. In U.S. Fish and Wildlife Service, Washington DC, Investigations in Fish Control No. 91, pp. 1-7. Cameron, J.A. 1940. Effect of flourine on hatching time and hatching stage in Rana pipens. Ecology 21(2):288-292.
Cameron, J.A. 1940. Effect of flourine on hatching time and hatching stage in Rana pipens. Ecology 21(2):288-292. Carey, C. 1993. Hypothesis concerning the causes of the disapperance of the boreal toads from the mountains of Colorado. Conservation Biology 7(2): 355-360.
Carey, C. 1993. Hypothesis concerning the causes of the disapperance of the boreal toads from the mountains of Colorado. Conservation Biology 7(2): 355-360. Carey, C., N. Cohen, and L. Rollins-Smith. 1999a. Amphibian declines: an immunological perspective. Developmental and Comparative Immunology 23: 459-472.
Carey, C., N. Cohen, and L. Rollins-Smith. 1999a. Amphibian declines: an immunological perspective. Developmental and Comparative Immunology 23: 459-472. Corkran, C.C. and C. Thoms. 2006. Amphibians of Oregon, Washington, and British Columbia. 2nd Edition. Vancouver, B.C.: Lone Pine Publishing. 176 p.
Corkran, C.C. and C. Thoms. 2006. Amphibians of Oregon, Washington, and British Columbia. 2nd Edition. Vancouver, B.C.: Lone Pine Publishing. 176 p. Corn, P.S. 1981. Field evidence for a relationship between color and developmental rate in the northern leopard frog (Rana pipiens). Herpetologica 37(3): 155-160.
Corn, P.S. 1981. Field evidence for a relationship between color and developmental rate in the northern leopard frog (Rana pipiens). Herpetologica 37(3): 155-160. Corn, P.S. and F.A. Vertucci. 1992. Descriptive risk assessment of the effects of acid deposition on Rocky Mountain Amphibians. Journal of Herpetology 26(4): 361-369.
Corn, P.S. and F.A. Vertucci. 1992. Descriptive risk assessment of the effects of acid deposition on Rocky Mountain Amphibians. Journal of Herpetology 26(4): 361-369. Corn, P.S. and J.C. Fogleman. 1984. Extinction of montane populations of the northern leopard frog (Rana pipiens) in Colorado. J. Herpetol. 18(2):147-152.
Corn, P.S. and J.C. Fogleman. 1984. Extinction of montane populations of the northern leopard frog (Rana pipiens) in Colorado. J. Herpetol. 18(2):147-152. Corn, P.S. and L.J. Livo. 1989. Leopard frog and wood frog reproduction in Colorado and Wyoming. Northwestern Naturalist 70:1-9.
Corn, P.S. and L.J. Livo. 1989. Leopard frog and wood frog reproduction in Colorado and Wyoming. Northwestern Naturalist 70:1-9. Cunjak, R.A. 1986. Winter habitat of northern leopard frogs (Rana pipiens) in a southern Ontario stream. Canadian Journal of Zoology 64: 255-257.
Cunjak, R.A. 1986. Winter habitat of northern leopard frogs (Rana pipiens) in a southern Ontario stream. Canadian Journal of Zoology 64: 255-257. Daszak, P., A.A. Cunningham and A.D. Hyatt. 2000. Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 287: 443-449.
Daszak, P., A.A. Cunningham and A.D. Hyatt. 2000. Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 287: 443-449. Daszak, P., L. Berger, A.A. Cunningham, A.D. Hyatt, D.E. Green, and R. Speare. 1999. Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases 5(6): 735-748.
Daszak, P., L. Berger, A.A. Cunningham, A.D. Hyatt, D.E. Green, and R. Speare. 1999. Emerging infectious diseases and amphibian population declines. Emerging Infectious Diseases 5(6): 735-748. Daugherty, C.H., L.N. Wishard, and L.B. Daugherty. 1978. Sexual dimorphism in an anuran response to severe thermal stress. Journal of Herpetology 12(3): 431-432.
Daugherty, C.H., L.N. Wishard, and L.B. Daugherty. 1978. Sexual dimorphism in an anuran response to severe thermal stress. Journal of Herpetology 12(3): 431-432. DeBenedictis, P.A. 1974. Interspecific competition betweeen tadpoles of Rana pipiens and Rana sylvatica: an experimental field study. Ecological Monographs 44: 129-151.
DeBenedictis, P.A. 1974. Interspecific competition betweeen tadpoles of Rana pipiens and Rana sylvatica: an experimental field study. Ecological Monographs 44: 129-151. Dial, N.A. and C.A. Baurer Dial. 1987. Lethal effects of diquat and paraquat on developing frog embryos and 15 -day-old tadpoles (Rana pipiens). Bulletin of Environmental Contamination and Toxicology 38: 1006-1011.
Dial, N.A. and C.A. Baurer Dial. 1987. Lethal effects of diquat and paraquat on developing frog embryos and 15 -day-old tadpoles (Rana pipiens). Bulletin of Environmental Contamination and Toxicology 38: 1006-1011. Dole, J.W. 1965a. Spatial relations in natural populations of the leopard frog (Rana pipiens) Schreber, in northern Michigan. American Midland Naturalist 74: 464-478.
Dole, J.W. 1965a. Spatial relations in natural populations of the leopard frog (Rana pipiens) Schreber, in northern Michigan. American Midland Naturalist 74: 464-478. Dole, J.W. 1965b. Summer movement of adult leopard frogs (Rana pipiens) Schreber, in northern Michigan. Ecology 46(3): 236-255.
Dole, J.W. 1965b. Summer movement of adult leopard frogs (Rana pipiens) Schreber, in northern Michigan. Ecology 46(3): 236-255. Dole, J.W. 1967a. Spring movements of leopard frogs (Rana pipiens) Schreber, in northern Michigan. American Midland Naturalist 78(1): 167-181.
Dole, J.W. 1967a. Spring movements of leopard frogs (Rana pipiens) Schreber, in northern Michigan. American Midland Naturalist 78(1): 167-181. Dole, J.W. 1967b. The role of substrate moisture and dew in the water economy of leopard frogs (Rana pipiens). Copeia 1967(1): 141-149.
Dole, J.W. 1967b. The role of substrate moisture and dew in the water economy of leopard frogs (Rana pipiens). Copeia 1967(1): 141-149. Dole, J.W. 1968. Homing in leopard frogs (Rana pipiens). Ecology 49(3): 386-399.
Dole, J.W. 1968. Homing in leopard frogs (Rana pipiens). Ecology 49(3): 386-399. Dole, J.W. 1971. Dispersal of recently metamorphosed leopard frogs (Rana pipiens). Copeia 1971(2): 221-228.
Dole, J.W. 1971. Dispersal of recently metamorphosed leopard frogs (Rana pipiens). Copeia 1971(2): 221-228. Dood, A.R. 1980. Terry Badlands nongame survey and inventory final report. Montana Department of Fish, Wildlife, and Parks and Bureau of Land Management, Helena, MT. 70 pp.
Dood, A.R. 1980. Terry Badlands nongame survey and inventory final report. Montana Department of Fish, Wildlife, and Parks and Bureau of Land Management, Helena, MT. 70 pp. Dumas, P.C. 1964. Species-pair allopatry in the genera Rana and Phrynosoma. Ecology 45(1): 178-181.
Dumas, P.C. 1964. Species-pair allopatry in the genera Rana and Phrynosoma. Ecology 45(1): 178-181. Dumas, P.C. 1966. Studies of the Rana species complex in the Pacific Northwest. Copeia 1966(1): 60-74.
Dumas, P.C. 1966. Studies of the Rana species complex in the Pacific Northwest. Copeia 1966(1): 60-74. Dunlap, D.G. and J.E. Platz. 1981. Geographic variation of proteins and call in Rana pipiens from the northcentral United States. Copeia 1981: 876-879.
Dunlap, D.G. and J.E. Platz. 1981. Geographic variation of proteins and call in Rana pipiens from the northcentral United States. Copeia 1981: 876-879. Emery, A.R., A.H. Berst, and K. Kodaira. 1972. Under-ice observations of wintering sites of leopard frogs. Copeia 1972(1): 123-126.
Emery, A.R., A.H. Berst, and K. Kodaira. 1972. Under-ice observations of wintering sites of leopard frogs. Copeia 1972(1): 123-126. Fogleman, J.C., P.S. Corn, and D. Pettus. 1980. The genetic basis of a dorsal color polymorphism in Rana pipiens. Journal of Heredity 71: 439-440.
Fogleman, J.C., P.S. Corn, and D. Pettus. 1980. The genetic basis of a dorsal color polymorphism in Rana pipiens. Journal of Heredity 71: 439-440. Freda, J. and W.A. Dunson. 1985. Field and laboratory studies of ion balance and growth rates of ranid tadpoles chronically exposed to low pH. Copeia 1985(2): 415-423.
Freda, J. and W.A. Dunson. 1985. Field and laboratory studies of ion balance and growth rates of ranid tadpoles chronically exposed to low pH. Copeia 1985(2): 415-423. Freda, J., V. Cavdek, and D.G. McDonald. 1990. Role of organic complexation in the toxicity of aluminum to Rana pipens embryos and Bufo americanus tadpoles. Canadian Journal of Fisheries and Aquatic Sciences 47: 217-224.
Freda, J., V. Cavdek, and D.G. McDonald. 1990. Role of organic complexation in the toxicity of aluminum to Rana pipens embryos and Bufo americanus tadpoles. Canadian Journal of Fisheries and Aquatic Sciences 47: 217-224. Freda, J., W.J. Sadinski, and W.A. Dunson. 1991. Long term monitoring of amphibian populations with respect to the effects of acidic deposition. Water, Air, and Soil Pollution 55: 445-462.
Freda, J., W.J. Sadinski, and W.A. Dunson. 1991. Long term monitoring of amphibian populations with respect to the effects of acidic deposition. Water, Air, and Soil Pollution 55: 445-462. Gilbert, M., R. Leclair, Jr. and R. Fortin. 1994. Reproduction of the northern leopard frog (Rana pipiens) in floodplain habitat in the Richelieu River, P. Quebec, Canada. Journal of Herpetology 28: 465-470.
Gilbert, M., R. Leclair, Jr. and R. Fortin. 1994. Reproduction of the northern leopard frog (Rana pipiens) in floodplain habitat in the Richelieu River, P. Quebec, Canada. Journal of Herpetology 28: 465-470. Hamilton, H.L. 1941. The biological action of rotenone on freshwater animals. Proceedings of the Iowa Academy of Science 48:467-479.
Hamilton, H.L. 1941. The biological action of rotenone on freshwater animals. Proceedings of the Iowa Academy of Science 48:467-479. Hammerson, G.A. 1982. Bullfrog eliminating leopard frogs in Colorado? Herpetological Review 13: 115-116.
Hammerson, G.A. 1982. Bullfrog eliminating leopard frogs in Colorado? Herpetological Review 13: 115-116. Hammerson, G.A. 1999. Amphibians and reptiles in Colorado. University Press of Colorado & Colorado Division of Wildlife. Denver, CO. 484 p.
Hammerson, G.A. 1999. Amphibians and reptiles in Colorado. University Press of Colorado & Colorado Division of Wildlife. Denver, CO. 484 p. Hecnar, S.J. 1995. Acute and chronic toxicity of ammonium nitrate fertilizer to amphibians from southern Ontario. Environmental Toxicology and Chemistry 14(12): 2131-2137.
Hecnar, S.J. 1995. Acute and chronic toxicity of ammonium nitrate fertilizer to amphibians from southern Ontario. Environmental Toxicology and Chemistry 14(12): 2131-2137. Hendricks, P. and J.D. Reichel. 1996a. Amphibian and reptile survey of the Bitterroot National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 95 p.
Hendricks, P. and J.D. Reichel. 1996a. Amphibian and reptile survey of the Bitterroot National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 95 p. Hendricks, P. and J.D. Reichel. 1996b. Preliminary amphibian and reptile survey of the Ashland District, Custer National Forest: 1995. Montana Natural Heritage Program, Helena, MT. 79 p.
Hendricks, P. and J.D. Reichel. 1996b. Preliminary amphibian and reptile survey of the Ashland District, Custer National Forest: 1995. Montana Natural Heritage Program, Helena, MT. 79 p. Hillis, D.M. 1988. Systematics of the Rana pipiens complex: puzzle and paradigm. Annual Review of Ecology and Systematics 19: 39-63.
Hillis, D.M. 1988. Systematics of the Rana pipiens complex: puzzle and paradigm. Annual Review of Ecology and Systematics 19: 39-63. Hossack, B., D. Pilliod, and S. Corn. 2003. Amphibian survey of Medicine Lake National Wildlife Complex: 2001-2002. USGS Northern Rocky Mountain Science Center, Aldo Leopold Wilderness Research Institute, Missoula, Montana. 19 p.
Hossack, B., D. Pilliod, and S. Corn. 2003. Amphibian survey of Medicine Lake National Wildlife Complex: 2001-2002. USGS Northern Rocky Mountain Science Center, Aldo Leopold Wilderness Research Institute, Missoula, Montana. 19 p. Kaplan, H.M. and J.G. Overpeck. 1964. Toxicity of halogenated hydrocarbon insecticides for the frog Rana pipiens. Herpetologica 20: 163-169.
Kaplan, H.M. and J.G. Overpeck. 1964. Toxicity of halogenated hydrocarbon insecticides for the frog Rana pipiens. Herpetologica 20: 163-169. Kaplan, H.M. and S.S. Glaczenski. 1965. Hematological effects of organophosphate insecticides in the frog Rana pipiens. Life Sciences 3: 1213-1219.
Kaplan, H.M. and S.S. Glaczenski. 1965. Hematological effects of organophosphate insecticides in the frog Rana pipiens. Life Sciences 3: 1213-1219. Karns, D. R. 1992. Effects of acidic bog habitats on amphibian reproduction in a northern Minnesota peatland. Journal of Herpetology 26:401-412.
Karns, D. R. 1992. Effects of acidic bog habitats on amphibian reproduction in a northern Minnesota peatland. Journal of Herpetology 26:401-412. Knowlton, G.F. 1944. Some insect food of Rana pipiens. Copeia 1944(2): 119.
Knowlton, G.F. 1944. Some insect food of Rana pipiens. Copeia 1944(2): 119. Koch, E.D. and C.R. Peterson. 1995. Amphibians and reptiles of Yellowstone and Grand Teton national parks. University of Utah Press, Salt Lake City, UT. 188 p.
Koch, E.D. and C.R. Peterson. 1995. Amphibians and reptiles of Yellowstone and Grand Teton national parks. University of Utah Press, Salt Lake City, UT. 188 p. Koch, E.D., G. Williams, C.R. Peterson and P.S. Corn. 1996. Conference on declining and sensitive amphibians in the Northern Rockies and the Pacific Northwest: a summary paper. Idaho Herpetological Society Technical Bulletin and U.S. Fish and Wildlife Service Report, Boise, Idaho.
Koch, E.D., G. Williams, C.R. Peterson and P.S. Corn. 1996. Conference on declining and sensitive amphibians in the Northern Rockies and the Pacific Northwest: a summary paper. Idaho Herpetological Society Technical Bulletin and U.S. Fish and Wildlife Service Report, Boise, Idaho. Lande, S.P. and S.I. Guttman. 1973. The effects of copper sulfate on the growth and mortality rate of Rana pipiens tadpoles. Herpetologica 29:22-27.
Lande, S.P. and S.I. Guttman. 1973. The effects of copper sulfate on the growth and mortality rate of Rana pipiens tadpoles. Herpetologica 29:22-27. Leonard, W.P. and K.R. McAllister. 1996. Past distribution and current status of the Northern Leopard frog (Rana pipiens) in Washington. Washington Department of Fish and Wildlife Special Publication, Feb. 1996. 17 p.
Leonard, W.P. and K.R. McAllister. 1996. Past distribution and current status of the Northern Leopard frog (Rana pipiens) in Washington. Washington Department of Fish and Wildlife Special Publication, Feb. 1996. 17 p. Leonard, W.P., K.R. McAllister, and R.C. Friesz. 1999. Survey and assessment of northern leopard frog (Rana pipiens) populations in Washington State. Northwestern Naturalist 80: 51-60.
Leonard, W.P., K.R. McAllister, and R.C. Friesz. 1999. Survey and assessment of northern leopard frog (Rana pipiens) populations in Washington State. Northwestern Naturalist 80: 51-60. Linzey, D.W. 1967. Food of the leopard frog (Rana p. pipiens) in Central New York. Herpetologica 23(1): 11-17
Linzey, D.W. 1967. Food of the leopard frog (Rana p. pipiens) in Central New York. Herpetologica 23(1): 11-17 Livezey, R.L. and A.H. Wright. 1947. A synoptic key to salientian eggs of the United States. American Midland Naturalist 37: 179-222.
Livezey, R.L. and A.H. Wright. 1947. A synoptic key to salientian eggs of the United States. American Midland Naturalist 37: 179-222. Long, L.E., L.S. Saylor, and M.E. Soule. 1995. A pH/UV-B synergism in amphibians. Conservation Biology 9(5): 1301-1303.
Long, L.E., L.S. Saylor, and M.E. Soule. 1995. A pH/UV-B synergism in amphibians. Conservation Biology 9(5): 1301-1303. Maniero, G.D. and C. Carey. 1997. Changes in selected aspects of immune function in the leopard frog (Rana pipiens) associated with exposure to cold. Journal of Comparative Physiology B 167: 256-263.
Maniero, G.D. and C. Carey. 1997. Changes in selected aspects of immune function in the leopard frog (Rana pipiens) associated with exposure to cold. Journal of Comparative Physiology B 167: 256-263. Maxell, B.A., J.K. Werner, P. Hendricks, and D.L. Flath. 2003. Herpetology in Montana: a history, status summary, checklists, dichotomous keys, accounts for native, potentially native, and exotic species, and indexed bibliography. Society for Northwestern Vertebrate Biology, Northwest Fauna Number 5. Olympia, WA. 135 p.
Maxell, B.A., J.K. Werner, P. Hendricks, and D.L. Flath. 2003. Herpetology in Montana: a history, status summary, checklists, dichotomous keys, accounts for native, potentially native, and exotic species, and indexed bibliography. Society for Northwestern Vertebrate Biology, Northwest Fauna Number 5. Olympia, WA. 135 p. Maxell, B.A., P. Hendricks, M.T. Gates, and S. Lenard. 2009. Montana amphibian and reptile status assessment, literature review, and conservation plan, June 2009. Montana Natural Heritage Program. Helena, MT. 643 p.
Maxell, B.A., P. Hendricks, M.T. Gates, and S. Lenard. 2009. Montana amphibian and reptile status assessment, literature review, and conservation plan, June 2009. Montana Natural Heritage Program. Helena, MT. 643 p. Milius, S. 1999a. Killer skin fungus nails boreal toads. Science News 156: 219.
Milius, S. 1999a. Killer skin fungus nails boreal toads. Science News 156: 219. Milius, S. 2000. New frog-killing disease may not be so new. Science News 157: 133.
Milius, S. 2000. New frog-killing disease may not be so new. Science News 157: 133. Miller, J. D. 1975. Interspecific food relationships of anurans in northwestern Montana and fluoride accumulation in amphibians and reptiles in northwestern Montana. M.S. thesis. University of Montana, Missoula, MT. 105 p.
Miller, J. D. 1975. Interspecific food relationships of anurans in northwestern Montana and fluoride accumulation in amphibians and reptiles in northwestern Montana. M.S. thesis. University of Montana, Missoula, MT. 105 p. Miller, J.D. 1978. Observations on the diets of Rana pretiosa, Rana pipiens, and Bufo boreas from western Montana. Northwest Science 52(3): 243-249.
Miller, J.D. 1978. Observations on the diets of Rana pretiosa, Rana pipiens, and Bufo boreas from western Montana. Northwest Science 52(3): 243-249. Moore, J.E. and E.H. Strickland. 1954. Notes on the food of three species of Alberta amphibians. American Midland Naturalist 52: 221-224.
Moore, J.E. and E.H. Strickland. 1954. Notes on the food of three species of Alberta amphibians. American Midland Naturalist 52: 221-224. Morell, V. 1999. Are pathogens felling frogs? Science 284: 728-731.
Morell, V. 1999. Are pathogens felling frogs? Science 284: 728-731. Mosimann, J.E. and G.B. Rabb. 1952. The herpetology of Tiber Reservoir Area, Montana. Copeia(1): 23-27.
Mosimann, J.E. and G.B. Rabb. 1952. The herpetology of Tiber Reservoir Area, Montana. Copeia(1): 23-27. Nash, R.F., G.G. Gallup, Jr. and M.K. McClure. 1970. The immobility reaction in leopard frogs (Rana pipiens) as a function of noise-induced fear. Psychonometric. Science 21(3): 155-156.
Nash, R.F., G.G. Gallup, Jr. and M.K. McClure. 1970. The immobility reaction in leopard frogs (Rana pipiens) as a function of noise-induced fear. Psychonometric. Science 21(3): 155-156. Nussbaum, R.A., E.D. Brodie, Jr. and R.M. Storm. 1983. Amphibians and reptiles of the Pacific Northwest. University of Idaho Press. Moscow, ID. 332 pp.
Nussbaum, R.A., E.D. Brodie, Jr. and R.M. Storm. 1983. Amphibians and reptiles of the Pacific Northwest. University of Idaho Press. Moscow, ID. 332 pp. Pace, A.E. 1974. Systematic and biological studies of the leopard frogs (Rana pipiens complex) of the United States. Miscellaneous Publications of Museum of Zoology University of Michigan 148.
Pace, A.E. 1974. Systematic and biological studies of the leopard frogs (Rana pipiens complex) of the United States. Miscellaneous Publications of Museum of Zoology University of Michigan 148. Rand, A.S. 1950. Leopard frogs in caves in winter. Copeia 1950(4): 324.
Rand, A.S. 1950. Leopard frogs in caves in winter. Copeia 1950(4): 324. Reichel, J.D. 1995a. Preliminary amphibian and reptile survey of the Lewis & Clark National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 92 p.
Reichel, J.D. 1995a. Preliminary amphibian and reptile survey of the Lewis & Clark National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 92 p. Reichel, J.D. 1995b. Preliminary amphibian and reptile survey of the Sioux District of the Custer National Forest: 1994. Montana Natural Heritage Program, Helena, MT. 75 p.
Reichel, J.D. 1995b. Preliminary amphibian and reptile survey of the Sioux District of the Custer National Forest: 1994. Montana Natural Heritage Program, Helena, MT. 75 p. Reichel, J.D. 1996. Preliminary amphibian and reptile survey of the Helena National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 87 pp.
Reichel, J.D. 1996. Preliminary amphibian and reptile survey of the Helena National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 87 pp. Roberts, W. 1987. The northern leopard frog - endangered in Alberta. Provincial Museum of Alberta Natural History Occassional Paper No. 9: 137-138.
Roberts, W. 1987. The northern leopard frog - endangered in Alberta. Provincial Museum of Alberta Natural History Occassional Paper No. 9: 137-138. Roberts, W.E. 1981. What happened to the leopard frogs? Alberta Naturalist 11: 1-4.
Roberts, W.E. 1981. What happened to the leopard frogs? Alberta Naturalist 11: 1-4. Roberts, W.E. 1991. An action plan for the recovery of the northern leopard frog in Alberta. Proceedings of the second endangered species and prairie conservation workshop. Provincial Musuem of Alberta, Natural History Occassional Paper No. 15 :199-200.
Roberts, W.E. 1991. An action plan for the recovery of the northern leopard frog in Alberta. Proceedings of the second endangered species and prairie conservation workshop. Provincial Musuem of Alberta, Natural History Occassional Paper No. 15 :199-200. Roberts, W.E. 1992. Declines in amphibian populations in Alberta. In: Declines in Canadian Amphibian Populations: Designing a National Monitoring Strategy, (Bishop, C.A. and K. E. Pettit, eds.), Occassional Paper 67, Canadian Wildlife Service.
Roberts, W.E. 1992. Declines in amphibian populations in Alberta. In: Declines in Canadian Amphibian Populations: Designing a National Monitoring Strategy, (Bishop, C.A. and K. E. Pettit, eds.), Occassional Paper 67, Canadian Wildlife Service. Roedel, M.D. and P. Hendricks. 1998a. Amphibian and reptile survey on the Bureau of Land Management Lewistown District: 1995-1998. Montana Natural Heritage Program, Helena, MT. 53 p.
Roedel, M.D. and P. Hendricks. 1998a. Amphibian and reptile survey on the Bureau of Land Management Lewistown District: 1995-1998. Montana Natural Heritage Program, Helena, MT. 53 p. Roedel, M.D. and P. Hendricks. 1998b. Amphibian and reptile inventory on the Headwaters and Dillon Resource Areas in conjunction with Red Rocks Lakes National Wildlife Refuge: 1996-1998. Montana Natural Heritage Program, Helena, MT. 46 p.
Roedel, M.D. and P. Hendricks. 1998b. Amphibian and reptile inventory on the Headwaters and Dillon Resource Areas in conjunction with Red Rocks Lakes National Wildlife Refuge: 1996-1998. Montana Natural Heritage Program, Helena, MT. 46 p. Ross, D.A., D.W. Kuehn and M.C. Stanger. 1994b. Rana pretiosa (spotted frog) reproduction. Herpetological Review 25(3): 118.
Ross, D.A., D.W. Kuehn and M.C. Stanger. 1994b. Rana pretiosa (spotted frog) reproduction. Herpetological Review 25(3): 118. Russell, A. P. and A. M. Bauer. 1993. The amphibians and reptiles of Alberta. University of Calgary Press. Calgary, Alberta. 264 p.
Russell, A. P. and A. M. Bauer. 1993. The amphibians and reptiles of Alberta. University of Calgary Press. Calgary, Alberta. 264 p. Ryan, S.A. 1980. Frog study of the Kicking Horse Reservoir on the Flathead Indian Reservation. Confederated Salish and Kootenai Tribal Wildlife Division, Pablo, MT. 3 p.
Ryan, S.A. 1980. Frog study of the Kicking Horse Reservoir on the Flathead Indian Reservation. Confederated Salish and Kootenai Tribal Wildlife Division, Pablo, MT. 3 p. Schlichter, L.C. 1981, Low pH affects the fertilization and development of Rana pipiens eggs. Canadian Journal of Zoology 59: 1963-1969.
Schlichter, L.C. 1981, Low pH affects the fertilization and development of Rana pipiens eggs. Canadian Journal of Zoology 59: 1963-1969. Seburn, C.N.L., D.C. Seburn, and C.A Paszkowski. 1997. Northern leopard frog (Rana pipiens) dispersal in relation to habitat. In: D.M. Green (ed.) Amphibians in decline: Canadian studies of a global problem. Society for the Study of Amphibians and Rep
Seburn, C.N.L., D.C. Seburn, and C.A Paszkowski. 1997. Northern leopard frog (Rana pipiens) dispersal in relation to habitat. In: D.M. Green (ed.) Amphibians in decline: Canadian studies of a global problem. Society for the Study of Amphibians and Rep Stebbins, R. C. 2003. A field guide to western reptiles and amphibians. 3rd Edition. Houghton Mifflin Company, Boston and New York. 533 p.
Stebbins, R. C. 2003. A field guide to western reptiles and amphibians. 3rd Edition. Houghton Mifflin Company, Boston and New York. 533 p. Stebbins, R.C. and N.W. Cohen. 1995. A natural history of amphibians. Princeton University Press. Princeton, NJ. 316 pp.
Stebbins, R.C. and N.W. Cohen. 1995. A natural history of amphibians. Princeton University Press. Princeton, NJ. 316 pp. Vatnick, I., M.A. Brodkin, M. Simon, B.W. Grant, C.R. Conte, M. Gleave, R. Myers, and M.M. Sadoff. 1999. The effects of exposure to mild acidic conditions on adult frogs (Rana pipiens and Rana clamitans): mortality arates and pH preferences. Journal of
Vatnick, I., M.A. Brodkin, M. Simon, B.W. Grant, C.R. Conte, M. Gleave, R. Myers, and M.M. Sadoff. 1999. The effects of exposure to mild acidic conditions on adult frogs (Rana pipiens and Rana clamitans): mortality arates and pH preferences. Journal of Wagner, G. 1997. Status of the northern leopard frog (Rana pipiens) in Alberta. Alberta Wildlife Status Report Number 9. 34 p.
Wagner, G. 1997. Status of the northern leopard frog (Rana pipiens) in Alberta. Alberta Wildlife Status Report Number 9. 34 p. Werner, J.K. and J.D. Reichel. 1994. Amphibian and reptile survey of the Kootenai National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 104 p.
Werner, J.K. and J.D. Reichel. 1994. Amphibian and reptile survey of the Kootenai National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 104 p. Werner, J.K. and J.D. Reichel. 1996. Amphibian and reptile monitoring/survey of the Kootenai National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 115 pp.
Werner, J.K. and J.D. Reichel. 1996. Amphibian and reptile monitoring/survey of the Kootenai National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 115 pp. Werner, J.K. and T. Plummer. 1995a. Amphibian and reptile survey of the Flathead Indian Reservation 1993-1994. Salish Kootenai College, Pablo, MT. 55 pp.
Werner, J.K. and T. Plummer. 1995a. Amphibian and reptile survey of the Flathead Indian Reservation 1993-1994. Salish Kootenai College, Pablo, MT. 55 pp. Werner, J.K. and T. Plummer. 1995b. Amphibian monitoring program on the Flathead Indian Reservation 1995. Salish Kootenai College, Pablo, MT. 46 p.
Werner, J.K. and T. Plummer. 1995b. Amphibian monitoring program on the Flathead Indian Reservation 1995. Salish Kootenai College, Pablo, MT. 46 p. Werner, J.K., B.A. Maxell, P. Hendricks and D.L. Flath. 2004. Amphibians and Reptiles of Montana. Mountain Press Publishing Company: Missoula, MT. 262 pp.
Werner, J.K., B.A. Maxell, P. Hendricks and D.L. Flath. 2004. Amphibians and Reptiles of Montana. Mountain Press Publishing Company: Missoula, MT. 262 pp. Werner, J.K., T. Plummer, and J. Weaslehead. 1998a. Amphibians and reptiles of the Flathead Indian Reservation. Intermountain Journal of Sciences 4(1-2): 33-49.
Werner, J.K., T. Plummer, and J. Weaslehead. 1998a. Amphibians and reptiles of the Flathead Indian Reservation. Intermountain Journal of Sciences 4(1-2): 33-49. Whitaker, J.A., Jr. 1961. Habitat and food of mouse-trapped young Rana pipiens and Rana clamitans. Herpetologica 17(3): 173-279.
Whitaker, J.A., Jr. 1961. Habitat and food of mouse-trapped young Rana pipiens and Rana clamitans. Herpetologica 17(3): 173-279.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? [DCC] Decker Coal Company. 1998. 1997 Consolidated annual progress report. Decker Coal Company West, North and East Pits. Decker, MT.
[DCC] Decker Coal Company. 1998. 1997 Consolidated annual progress report. Decker Coal Company West, North and East Pits. Decker, MT. [EI] Econ Incorporated. 1984. Terrestrial wildlife inventory for the Lame Jones and Ismay coal lease tracts. Econ Incorporated. Helena, MT.
[EI] Econ Incorporated. 1984. Terrestrial wildlife inventory for the Lame Jones and Ismay coal lease tracts. Econ Incorporated. Helena, MT. [OEA] Olson Elliot and Associates Research. 1985. 1983-1984 Wildlife monitoring report for the CX Ranch project. Olson Elliot and Associates Research. Helena, MT.
[OEA] Olson Elliot and Associates Research. 1985. 1983-1984 Wildlife monitoring report for the CX Ranch project. Olson Elliot and Associates Research. Helena, MT. [PRESI] Powder River Eagle Studies Incorporated. 1998a. Big Sky Mine 1997 wildlife monitoring studies. Powder River Eagle Studies Incorporated. Gillete, WY.
[PRESI] Powder River Eagle Studies Incorporated. 1998a. Big Sky Mine 1997 wildlife monitoring studies. Powder River Eagle Studies Incorporated. Gillete, WY. [PRESI] Powder River Eagle Studies Incorporated. 1998b. Spring Creek Mine 1997 wildlife monitoring studies. Powder River Eagle Studies Incorporated. Gillete, WY.
[PRESI] Powder River Eagle Studies Incorporated. 1998b. Spring Creek Mine 1997 wildlife monitoring studies. Powder River Eagle Studies Incorporated. Gillete, WY. [VTNWI] VTN Wyoming Incorporated. No Date. Second year's analysis of terrestrial wildlife on proposed mine access and railroad routes in southern Montana and northern Wyoming, March 1979 - February 1980. VTN Wyoming Incorporated. Sheridan, WY. 62 p.
[VTNWI] VTN Wyoming Incorporated. No Date. Second year's analysis of terrestrial wildlife on proposed mine access and railroad routes in southern Montana and northern Wyoming, March 1979 - February 1980. VTN Wyoming Incorporated. Sheridan, WY. 62 p. [WESCO] Western Ecological Services Company. 1983a. Wildlife inventory of the Knowlton known recoverable coal resource area, Montana. Western Ecological Services Company, Novato, CA. 107 p.
[WESCO] Western Ecological Services Company. 1983a. Wildlife inventory of the Knowlton known recoverable coal resource area, Montana. Western Ecological Services Company, Novato, CA. 107 p. [WESCO] Western Ecological Services Company. 1983b. Wildlife inventory of the Southwest Circle known recoverable coal resource area, Montana. Western Ecological Services Company, Novato, CA. 131 p.
[WESCO] Western Ecological Services Company. 1983b. Wildlife inventory of the Southwest Circle known recoverable coal resource area, Montana. Western Ecological Services Company, Novato, CA. 131 p. [WESTECH] Western Technology and Engineering Incorporated. 1991. Update On the Wildlife Resources of the Little Rocky Mountains Environmental Study Area. Western Technology and Engineering, Inc., Helena, Mt.
[WESTECH] Western Technology and Engineering Incorporated. 1991. Update On the Wildlife Resources of the Little Rocky Mountains Environmental Study Area. Western Technology and Engineering, Inc., Helena, Mt. [WESTECH] Western Technology and Engineering Incorporated. 1998. Wildlife Monitoring Absaloka Mine Area 1997. Western Technology and Engineering, Inc., Helena, Mt.
[WESTECH] Western Technology and Engineering Incorporated. 1998. Wildlife Monitoring Absaloka Mine Area 1997. Western Technology and Engineering, Inc., Helena, Mt. [WWPC] Washington Water Power Company. 1995. 1994 wildlife report Noxon Rapids and Cabinet Gorge Reservoirs. Washington Water Power Company. Spokane, WA.
[WWPC] Washington Water Power Company. 1995. 1994 wildlife report Noxon Rapids and Cabinet Gorge Reservoirs. Washington Water Power Company. Spokane, WA. Adama, D.B., M.A. Beaucher, and K. Lansley. 2002. Northern Leopard frogs in British Columbia--towards recovery. Northwestern Naturalist 83:62.
Adama, D.B., M.A. Beaucher, and K. Lansley. 2002. Northern Leopard frogs in British Columbia--towards recovery. Northwestern Naturalist 83:62. Allen, J. A. 1874. Notes on the natural history of portions of Dakota and Montana Territories, being the substance of a report to the Secretary of War on the collections made by the North Pacific Railroad Expedition of 1873. Proceedings of the Boston Society of Natural History. pp. 68-70.
Allen, J. A. 1874. Notes on the natural history of portions of Dakota and Montana Territories, being the substance of a report to the Secretary of War on the collections made by the North Pacific Railroad Expedition of 1873. Proceedings of the Boston Society of Natural History. pp. 68-70. Allran, J.W. and W.H. Karasov. 2000. Effects of atrazine and nitrate on northern leopard frog (Rana pipiens) larvae exposed in the laboratory from posthatch through metamorphosis. Environmental Toxicology and Chemistry 19(11): 2850-2855.
Allran, J.W. and W.H. Karasov. 2000. Effects of atrazine and nitrate on northern leopard frog (Rana pipiens) larvae exposed in the laboratory from posthatch through metamorphosis. Environmental Toxicology and Chemistry 19(11): 2850-2855. Amish, S.J. 2006. Ecosystem engineering: beaver and the population structure of Columbia spotted frogs in western Montana. M.S. Thesis. University of Montana. Missoula, MT. 96 p.
Amish, S.J. 2006. Ecosystem engineering: beaver and the population structure of Columbia spotted frogs in western Montana. M.S. Thesis. University of Montana. Missoula, MT. 96 p. Ankley, G.T., J.E. Tietge, D.L. DeFoe, K.M. Jensen, G.W. Holcombe, E.J. Durhan, and S.A. Diamond. 1998. Effects of ultraviolet light and methoprene on survival and development of Rana pipiens. Environmental Toxicology and Chemistry 17: 2530-2542.
Ankley, G.T., J.E. Tietge, D.L. DeFoe, K.M. Jensen, G.W. Holcombe, E.J. Durhan, and S.A. Diamond. 1998. Effects of ultraviolet light and methoprene on survival and development of Rana pipiens. Environmental Toxicology and Chemistry 17: 2530-2542. Ankley, G.T., S.A. Diamond, J.E. Tietge, G.W. Holcombe, K.M. Jensen, D.L. DeFoe, and R. Peterson. 2002. Assessment of the risk of solar ultraviolet radiation to amphibians. I. dose-dependent induction of hindlimb malformations in the northern leopard frog (Rana pipiens). Environmental Science and Technology 36(13): 2853-2858.
Ankley, G.T., S.A. Diamond, J.E. Tietge, G.W. Holcombe, K.M. Jensen, D.L. DeFoe, and R. Peterson. 2002. Assessment of the risk of solar ultraviolet radiation to amphibians. I. dose-dependent induction of hindlimb malformations in the northern leopard frog (Rana pipiens). Environmental Science and Technology 36(13): 2853-2858. Atkinson, E.C. and M.L. Atkinson. 2004. Amphibian and reptile survey of the Ashland and Sioux of the Custer National Forest with special emphasis on the Three-Mile Stewardship Area:2002. Marmot's Edge Conservation. 22 p.
Atkinson, E.C. and M.L. Atkinson. 2004. Amphibian and reptile survey of the Ashland and Sioux of the Custer National Forest with special emphasis on the Three-Mile Stewardship Area:2002. Marmot's Edge Conservation. 22 p. Bailey, M. 2004. Northern leopard frogs in a golf course water hazard. Blue Jay 62(1):43-45.
Bailey, M. 2004. Northern leopard frogs in a golf course water hazard. Blue Jay 62(1):43-45. Bauer, D. 1997. 1997 wildlife study Savage Mine report. Knife River Corporation, Savage Mine. Richland County, MT.
Bauer, D. 1997. 1997 wildlife study Savage Mine report. Knife River Corporation, Savage Mine. Richland County, MT. Baxter, G.T. 1952a. Notes on growth and the reproductive cycle of the leopard frog (Rana pipiens) Schreber, in southern Wyoming. Journal of the Colorado-Wyoming Academy of Science 4: 91.
Baxter, G.T. 1952a. Notes on growth and the reproductive cycle of the leopard frog (Rana pipiens) Schreber, in southern Wyoming. Journal of the Colorado-Wyoming Academy of Science 4: 91. Beauregard, N. and R. Leclair. 1988. Multivariate analysis of the summe habitat structure of Rana pipiens Schreber, in Lac Saint Pierre (Quebec a Trois Riviere, Quebec, Canada). Pages 129-143 R.C. Szaro, K.E. Severson, and D.R. Patton (eds.), Managemen
Beauregard, N. and R. Leclair. 1988. Multivariate analysis of the summe habitat structure of Rana pipiens Schreber, in Lac Saint Pierre (Quebec a Trois Riviere, Quebec, Canada). Pages 129-143 R.C. Szaro, K.E. Severson, and D.R. Patton (eds.), Managemen Beiswenger, R.E. 1988. Integrating anuran amphibian species into environmental assessment programs. Pages 159-165 in Szaro, R.C.//Severson, K.E.//Patton, D.R., technical coordinators. Management of amphibians, reptiles, and small mammals in North America. U.S. Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado. General Technical Report RM-166.
Beiswenger, R.E. 1988. Integrating anuran amphibian species into environmental assessment programs. Pages 159-165 in Szaro, R.C.//Severson, K.E.//Patton, D.R., technical coordinators. Management of amphibians, reptiles, and small mammals in North America. U.S. Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado. General Technical Report RM-166. Bergeron, D. 1978. Terrestrial Wildlife Survey Coal creek Mine Area, Montana. Unpublished report for Coal Creek Mining Co., Ashland, Montana.
Bergeron, D. 1978. Terrestrial Wildlife Survey Coal creek Mine Area, Montana. Unpublished report for Coal Creek Mining Co., Ashland, Montana. Bergeron, D.J. 1978a. Terrestrial wildlife survey Divide Mine area, Montana 1977-1978. Western Technology and Engineering, Inc. Helena, MT.
Bergeron, D.J. 1978a. Terrestrial wildlife survey Divide Mine area, Montana 1977-1978. Western Technology and Engineering, Inc. Helena, MT. Bergeron, D.J. 1978b. Terrestrial wildlife survey P-M Mine area, Montana 1977-1978. Western Technology and Engineering, Inc. Helena, MT.
Bergeron, D.J. 1978b. Terrestrial wildlife survey P-M Mine area, Montana 1977-1978. Western Technology and Engineering, Inc. Helena, MT. Bernstein, G.S. 1952. Sperm aggutination in the egg jelly of the frogs Rana pipiens, Scherber and R. clamitans, Latreille. Biology Bulletin 103:285.
Bernstein, G.S. 1952. Sperm aggutination in the egg jelly of the frogs Rana pipiens, Scherber and R. clamitans, Latreille. Biology Bulletin 103:285. BLM. 1982b. Moorhead baseline inventory - wildlife. Bureau of Land Management, Miles City District Office. Miles City, MT. 29 pp.
BLM. 1982b. Moorhead baseline inventory - wildlife. Bureau of Land Management, Miles City District Office. Miles City, MT. 29 pp. Blouin, M. 2001. Microsatellite DNA testing of Columbia spotted frog toe samples. Unpublished report. On file with: USDA Forest Service, Pacific Northwest Research Station, 1401 Gekeler Lane, La Grande, OR 97850.
Blouin, M. 2001. Microsatellite DNA testing of Columbia spotted frog toe samples. Unpublished report. On file with: USDA Forest Service, Pacific Northwest Research Station, 1401 Gekeler Lane, La Grande, OR 97850. Bodley, K.T. 2003. The effects of the presence of dragonfly predators on the morphology of the Columbia spotted frog (Rana luteiventris). Undergraduate Honors Thesis. Carroll College, Helena, MT. 13 p.
Bodley, K.T. 2003. The effects of the presence of dragonfly predators on the morphology of the Columbia spotted frog (Rana luteiventris). Undergraduate Honors Thesis. Carroll College, Helena, MT. 13 p. Bos, D.H. 2000. Conservation genetics and phylogeography of the Columbia spotted frog (Rana luteiventris). M.S. Thesis. Department of Zoology, Brigham Young University. Provo, Utah. 42 p.
Bos, D.H. 2000. Conservation genetics and phylogeography of the Columbia spotted frog (Rana luteiventris). M.S. Thesis. Department of Zoology, Brigham Young University. Provo, Utah. 42 p. Bos, D.H. and J.W. Sites, Jr. 2001. Phylogeography and conservation genetics of the Columbia spotted frog (Rana luteiventris; Amphibia, Ranidae). Molecular Ecology 10:1499-1513.
Bos, D.H. and J.W. Sites, Jr. 2001. Phylogeography and conservation genetics of the Columbia spotted frog (Rana luteiventris; Amphibia, Ranidae). Molecular Ecology 10:1499-1513. Boundy, J. 2001. Herpetofaunal surveys in the Clark Fork Valley region, Montana. Herpetological Natural History 8: 15-26.
Boundy, J. 2001. Herpetofaunal surveys in the Clark Fork Valley region, Montana. Herpetological Natural History 8: 15-26. Bovbjerg, R.V. 1965. Experimental studies on the dispersal of the frog (Rana pipiens). Proceedings of the Iowa Academy of Science 72: 412-418.
Bovbjerg, R.V. 1965. Experimental studies on the dispersal of the frog (Rana pipiens). Proceedings of the Iowa Academy of Science 72: 412-418. Bovbjerg, R.V. and A.M. Bovbjerg. 1964. Summer emigrations of the frog (Rana pipiens) in northwestern Iowa. Proceedings of the Iowa Academy of Science 71: 511-518.
Bovbjerg, R.V. and A.M. Bovbjerg. 1964. Summer emigrations of the frog (Rana pipiens) in northwestern Iowa. Proceedings of the Iowa Academy of Science 71: 511-518. Bramblett, R.G., and A.V. Zale. 2002. Montana Prairie Riparian Native Species Report. Montana Cooperative Fishery Research Unit, Montana State University - Bozeman.
Bramblett, R.G., and A.V. Zale. 2002. Montana Prairie Riparian Native Species Report. Montana Cooperative Fishery Research Unit, Montana State University - Bozeman. Bresler, J.B. 1964. Pigmentation characteristics of Rana pipiens: Tympanum spot, line on upper jaw, and spots on upper eyelids. American Midland Naturalist 72: 382-389.
Bresler, J.B. 1964. Pigmentation characteristics of Rana pipiens: Tympanum spot, line on upper jaw, and spots on upper eyelids. American Midland Naturalist 72: 382-389. Brodkin, M., I. Vatnick, M. Simon, H. Hopey, K. Butler-Holston, and M. Leonard. 2003. Effects of acid stress in adult Rana pipiens. Journal of Experimental Zoology 298A(1):16-22.
Brodkin, M., I. Vatnick, M. Simon, H. Hopey, K. Butler-Holston, and M. Leonard. 2003. Effects of acid stress in adult Rana pipiens. Journal of Experimental Zoology 298A(1):16-22. Brodkin, M.A., M.P. Simon, A.M. DeSantis and K.J. Boyer. 1992. Response of Rana pipiens to graded doses of the bacterium Pseudomonas aeruginosa. Journal of Herpetology 26(4): 490-495.
Brodkin, M.A., M.P. Simon, A.M. DeSantis and K.J. Boyer. 1992. Response of Rana pipiens to graded doses of the bacterium Pseudomonas aeruginosa. Journal of Herpetology 26(4): 490-495. Browder, L.W. 1967a. Pigmentation in Rana pipiens: a study of spotting patterns in the leopard frog. Ph.D. Diss., University of Minnesota.
Browder, L.W. 1967a. Pigmentation in Rana pipiens: a study of spotting patterns in the leopard frog. Ph.D. Diss., University of Minnesota. Browder, L.W. and J. Davison. 1964. Spotting variations in the leopard frog: A test for the genetic basis in the Rana pipiens “burnsi” variant. Journal of Heredity 55: 234-241.
Browder, L.W. and J. Davison. 1964. Spotting variations in the leopard frog: A test for the genetic basis in the Rana pipiens “burnsi” variant. Journal of Heredity 55: 234-241. Browder. L.W. 1967b. Pigmentation in Rana pipiens. I. Inheritance of the speckle mutation. Journal of Heredity 52: 301-304.
Browder. L.W. 1967b. Pigmentation in Rana pipiens. I. Inheritance of the speckle mutation. Journal of Heredity 52: 301-304. Brown, L. E. and J. R. Brown. 1972. Call types of the Rana pipiens complex in Illinois. Science 176:928-929.
Brown, L. E. and J. R. Brown. 1972. Call types of the Rana pipiens complex in Illinois. Science 176:928-929. Brown, L.E. 1973. Speciation in the Rana pipiens complex. American Zoologist 13: 73-79.
Brown, L.E. 1973. Speciation in the Rana pipiens complex. American Zoologist 13: 73-79. Brown, L.E. 1992. Rana blairi. Catalogue of American Amphibians and Reptiles 536: 1-6.
Brown, L.E. 1992. Rana blairi. Catalogue of American Amphibians and Reptiles 536: 1-6. Brunson, R.B. 1955. Check list of the amphibians and reptiles of Montana. Proceedings of the Montana Academy of Sciences 15: 27-29.
Brunson, R.B. 1955. Check list of the amphibians and reptiles of Montana. Proceedings of the Montana Academy of Sciences 15: 27-29. Bull, E.L. 2000. Comparisons of two radio transmitter attachments on Columbia spotted frogs (Rana luteiventris). Herpetological Review 31(1): 26-28.
Bull, E.L. 2000. Comparisons of two radio transmitter attachments on Columbia spotted frogs (Rana luteiventris). Herpetological Review 31(1): 26-28. Bull, E.L. 2003. Diet and prey availability of Columbia spotted frogs in northeastern Oregon. Northwest Science 77:349-356.
Bull, E.L. 2003. Diet and prey availability of Columbia spotted frogs in northeastern Oregon. Northwest Science 77:349-356. Bull, E.L. 2005. Ecology of the Columbia spotted frog in northeastern Oregon. General Technical Report PNW-GTR-640. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. Portland, OR. 46 p.
Bull, E.L. 2005. Ecology of the Columbia spotted frog in northeastern Oregon. General Technical Report PNW-GTR-640. U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. Portland, OR. 46 p. Bull, E.L. and J.F. Shepherd. 2003. Water temperature at oviposition sites of Rana luteiventris in northeastern oregon. Western North American Naturalist 63(1): 108-113.
Bull, E.L. and J.F. Shepherd. 2003. Water temperature at oviposition sites of Rana luteiventris in northeastern oregon. Western North American Naturalist 63(1): 108-113. Bull, E.L. and M.P. Hayes. 2000. Livestock effects on reproduction of the Columbia spotted frog. Journal of Range Management 53: 293-296.
Bull, E.L. and M.P. Hayes. 2000. Livestock effects on reproduction of the Columbia spotted frog. Journal of Range Management 53: 293-296. Bull, E.L. and M.P. Hayes. 2001. Post-breeding season movements of Columbia spotted frogs (Rana luteiventris) in northeastern Oregon. Western North American Naturalist 61:119-123.
Bull, E.L. and M.P. Hayes. 2001. Post-breeding season movements of Columbia spotted frogs (Rana luteiventris) in northeastern Oregon. Western North American Naturalist 61:119-123. Bull, E.L. and M.P. Hayes. 2002. Overwintering of Columbia spotted frogs in northeastern Oregon. Northwest Science 76:141-147.
Bull, E.L. and M.P. Hayes. 2002. Overwintering of Columbia spotted frogs in northeastern Oregon. Northwest Science 76:141-147. Bureau of Indian Affairs, Department of the Interior. 1981. Draft Environmental Impact Statement. Unpublished report for the Crow/Shell Coal Lease, Crow Indian Reservation, Montana.
Bureau of Indian Affairs, Department of the Interior. 1981. Draft Environmental Impact Statement. Unpublished report for the Crow/Shell Coal Lease, Crow Indian Reservation, Montana. Burton, S.R., D.A. Patla, and C.R. Peterson. 2002. Amphibians of Red Rock Lakes National Wildlife Refuge: occurrence, distribution, relative abundance, and habitat associations. Herpetology Laboratory, Department of Biological Sciences, Idaho State University, Pocatello, ID. 66 p.
Burton, S.R., D.A. Patla, and C.R. Peterson. 2002. Amphibians of Red Rock Lakes National Wildlife Refuge: occurrence, distribution, relative abundance, and habitat associations. Herpetology Laboratory, Department of Biological Sciences, Idaho State University, Pocatello, ID. 66 p. Butts, T.W. 1997. Mountain Inc. wildlife monitoring Bull Mountains Mine No. 1, 1996. Western Technology and Engineering. Helena, MT.
Butts, T.W. 1997. Mountain Inc. wildlife monitoring Bull Mountains Mine No. 1, 1996. Western Technology and Engineering. Helena, MT. Carey, C. 1979a. Aerobic and anaerobic energy expenditure during rest and activity in montane Bufo b. boreas and Rana pipiens. Oecologia 39: 213-228.
Carey, C. 1979a. Aerobic and anaerobic energy expenditure during rest and activity in montane Bufo b. boreas and Rana pipiens. Oecologia 39: 213-228. Carlsen, T. and R. Northrup. 1992. Canyon Ferry Wildlife Management Area Final Draft Management Plan. March 1992.
Carlsen, T. and R. Northrup. 1992. Canyon Ferry Wildlife Management Area Final Draft Management Plan. March 1992. Carlson, J. (Coordinator, Montana Animal Species of Concern Committee). 2003. Montana Animal Species of Concern January 2003. Helena, MT: Montana Natural Heritage Program and Montana Fish, Wildlife, and Parks. In Press. 12p.
Carlson, J. (Coordinator, Montana Animal Species of Concern Committee). 2003. Montana Animal Species of Concern January 2003. Helena, MT: Montana Natural Heritage Program and Montana Fish, Wildlife, and Parks. In Press. 12p. Clarkson, R.W. and J.C. Rorabaugh. 1989. Status of leopard frogs (Rana pipiens complex: Ranidae) in Arizona and southeastern California. Southwestern Naturalist 34: 531-538.
Clarkson, R.W. and J.C. Rorabaugh. 1989. Status of leopard frogs (Rana pipiens complex: Ranidae) in Arizona and southeastern California. Southwestern Naturalist 34: 531-538. Clarkson, R.W. and J.C. Rorabaugh. 1989. Status of the leopard frogs (Rana pipiens complex: Ranidae) in Arizona and southwestern California. Southwestern Naturalist 34: 531-538.
Clarkson, R.W. and J.C. Rorabaugh. 1989. Status of the leopard frogs (Rana pipiens complex: Ranidae) in Arizona and southwestern California. Southwestern Naturalist 34: 531-538. Cobell, B. and R. Wagner. 2002. An evaluation of the terrestrial and aquatic resources of Malmstrom Air Force Base. USFWS - Montana Fish and Wildlife Management Assistance Office. 28 pgs + append.
Cobell, B. and R. Wagner. 2002. An evaluation of the terrestrial and aquatic resources of Malmstrom Air Force Base. USFWS - Montana Fish and Wildlife Management Assistance Office. 28 pgs + append. Cochran, D.C. 1961. Type specimens of reptiles and amphibians in the United States National Museum. U.S. National Museum Bulletin (220) xv + 291pp.
Cochran, D.C. 1961. Type specimens of reptiles and amphibians in the United States National Museum. U.S. National Museum Bulletin (220) xv + 291pp. Collins, J.P. and M.A. Lewis. 1979. Overwintering tadpoles and breeding season variation in the Rana pipiens complex in Arizona. Southwestern Naturalist 24(2): 371-396.
Collins, J.P. and M.A. Lewis. 1979. Overwintering tadpoles and breeding season variation in the Rana pipiens complex in Arizona. Southwestern Naturalist 24(2): 371-396. Confluence Consulting Inc. 2010. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT.
Confluence Consulting Inc. 2010. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT. Confluence Consulting Inc. 2011. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT.
Confluence Consulting Inc. 2011. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT. Confluence Consulting Inc. 2012. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT.
Confluence Consulting Inc. 2012. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT. Confluence Consulting Inc. 2013. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT.
Confluence Consulting Inc. 2013. Montana Department of Transportation Wetland Mitigation Monitoring Reports (various sites). MDT Helena, MT. Cooper, J. G. 1869. Notes on the fauna of the Upper Missouri. American Naturalist 3(6):294-299.
Cooper, J. G. 1869. Notes on the fauna of the Upper Missouri. American Naturalist 3(6):294-299. Cooper, S.V., C. Jean, and P. Hendricks. 2001. Biological survey of a prairie landscape in Montana's glaciated plains. Report to the Bureau of Land Management. Montana Natural Heritage Program, Helena, MT. 24 pp. plus appendices.
Cooper, S.V., C. Jean, and P. Hendricks. 2001. Biological survey of a prairie landscape in Montana's glaciated plains. Report to the Bureau of Land Management. Montana Natural Heritage Program, Helena, MT. 24 pp. plus appendices. Cope, E. D. 1879. A contribution to the zoology of Montana. American Naturalist 13(7): 432-441.
Cope, E. D. 1879. A contribution to the zoology of Montana. American Naturalist 13(7): 432-441. Cope, E.D. 1875. Check-list of North American Batrachia and Reptilia; with a systematic list of the higher groups, and an essay on geographical distribution. Based on the specimens contained in the U.S. National Museum. U.S. Natioanl Museum Bulletin 1: 1-104.
Cope, E.D. 1875. Check-list of North American Batrachia and Reptilia; with a systematic list of the higher groups, and an essay on geographical distribution. Based on the specimens contained in the U.S. National Museum. U.S. Natioanl Museum Bulletin 1: 1-104. Cope, E.D. 1889. The Batrachia of North America. Bulletin of the U.S. National Museum 34: 1-525, figs. 1-119, pls. 1-86.
Cope, E.D. 1889. The Batrachia of North America. Bulletin of the U.S. National Museum 34: 1-525, figs. 1-119, pls. 1-86. Corcoran, M.F and J. Travis. 1980. A comparison of the karyotypes of the frogs Rana areolata, Rana sphenocephala and Rana pipiens. Herpetologica 36: 296-300.
Corcoran, M.F and J. Travis. 1980. A comparison of the karyotypes of the frogs Rana areolata, Rana sphenocephala and Rana pipiens. Herpetologica 36: 296-300. Corn, P.S. 1982. Selection pressures affecting a dorsal color polymorphism in Rana pipiens. Ph.D. Dissertation. Colorado State University. Fort Collins, Colorado.
Corn, P.S. 1982. Selection pressures affecting a dorsal color polymorphism in Rana pipiens. Ph.D. Dissertation. Colorado State University. Fort Collins, Colorado. Corn, P.S., E. Muths, and W.M. Iko. 2000. A comparison in Colorado of three methods to monitor breeding amphibians. Northwestern Naturalist 81:22-30.
Corn, P.S., E. Muths, and W.M. Iko. 2000. A comparison in Colorado of three methods to monitor breeding amphibians. Northwestern Naturalist 81:22-30. Corn, P.S., M.L. Jennings, and E. Muths. 1997. Survey and assessment of amphibian populations in Rocky Mountain National Park. Northwest. Nat. 78:34-55.
Corn, P.S., M.L. Jennings, and E. Muths. 1997. Survey and assessment of amphibian populations in Rocky Mountain National Park. Northwest. Nat. 78:34-55. Corn, S. 1979. Size at metamorphosis and growth rates of juvenile frogs from montane populations of Rana pipiens. Journal of the Colorado-Wyoming Academy of Science 11(1): 90.
Corn, S. 1979. Size at metamorphosis and growth rates of juvenile frogs from montane populations of Rana pipiens. Journal of the Colorado-Wyoming Academy of Science 11(1): 90. Coues, E. and H. Yarrow. 1878. Notes on the herpetology of Dakota and Montana. Bulletin of the U.S. Geological Geographic Survey of the Territories 4: 259-291.
Coues, E. and H. Yarrow. 1878. Notes on the herpetology of Dakota and Montana. Bulletin of the U.S. Geological Geographic Survey of the Territories 4: 259-291. Cousineau, M. and K. Rogers. 1991. Observations on sympatric Rana pipiens, R. blairi, and their hybrids in eastern Colorado. Journal of Herpetology 25: 114-116.
Cousineau, M. and K. Rogers. 1991. Observations on sympatric Rana pipiens, R. blairi, and their hybrids in eastern Colorado. Journal of Herpetology 25: 114-116. Croshaw, D.A. 2005. Cryptic behavior is independent of dorsal color polymorphism in juvenile northern leopard frogs (Rana pipiens). Journal of Herpetology 39(1):125-129.
Croshaw, D.A. 2005. Cryptic behavior is independent of dorsal color polymorphism in juvenile northern leopard frogs (Rana pipiens). Journal of Herpetology 39(1):125-129. Crother, B.I. (ed.) 2008. Scientific and standard English names of amphibians and reptiles of North America north of Mexico. SSAR Herpetological Circular No. 37:1-84.
Crother, B.I. (ed.) 2008. Scientific and standard English names of amphibians and reptiles of North America north of Mexico. SSAR Herpetological Circular No. 37:1-84. Cuellar, H.S. 1971. Levels of genetic compatibility of Rana areolata with southwestern members of the Rana pipiens complex (Anura: Ranidae). Evolution 25: 399-409.
Cuellar, H.S. 1971. Levels of genetic compatibility of Rana areolata with southwestern members of the Rana pipiens complex (Anura: Ranidae). Evolution 25: 399-409. Davidson, J. 1961. A study of spotting patterns in the leopard frog. Journal of Heredity 52: 301-304.
Davidson, J. 1961. A study of spotting patterns in the leopard frog. Journal of Heredity 52: 301-304. Davis, A.B. and P.A. Verrell. 2005. Demography and reproductive ecology of the Columbia spotted frog (Rana luteiventris) across the Palouse. Canadian Journal of Zoology 83(5):702-711.
Davis, A.B. and P.A. Verrell. 2005. Demography and reproductive ecology of the Columbia spotted frog (Rana luteiventris) across the Palouse. Canadian Journal of Zoology 83(5):702-711. Day, D. 1989. Montco Terrestrial Wildlife Monitoring Report. Unpublished report for Montco, Billings, Montana.
Day, D. 1989. Montco Terrestrial Wildlife Monitoring Report. Unpublished report for Montco, Billings, Montana. Day, D., P.J. Farmer, and C.E. Farmer. 1989. Montco terrestrial wildlife monitoring report December, 1987 - July, 1989. Montco, Billings, MT, and Western Technology and Engineering, Inc. Helena, MT.
Day, D., P.J. Farmer, and C.E. Farmer. 1989. Montco terrestrial wildlife monitoring report December, 1987 - July, 1989. Montco, Billings, MT, and Western Technology and Engineering, Inc. Helena, MT. Di Berrardino, M.A. 1962. The karyotype of Rana pipiens and investigation of its stability during embryonic differentiation. Developmental Biology 5: 101-126.
Di Berrardino, M.A. 1962. The karyotype of Rana pipiens and investigation of its stability during embryonic differentiation. Developmental Biology 5: 101-126. Dole, J.W. 1963. Movements and spatial relations of Rana pipiens in spring and summer in northern Michigan. Ph.D. Thesis, University of Michigan, Ann Arbor. 288p.
Dole, J.W. 1963. Movements and spatial relations of Rana pipiens in spring and summer in northern Michigan. Ph.D. Thesis, University of Michigan, Ann Arbor. 288p. Dole, J.W. 1972. The role of olfaction and audition in the orientation of leopard frogs (Rana pipiens). Herpetologica 28(3): 258-260.
Dole, J.W. 1972. The role of olfaction and audition in the orientation of leopard frogs (Rana pipiens). Herpetologica 28(3): 258-260. Drake, C.J. 1914. The food of Rana pipiens Schreber. Ohio Naturalist 14: 257-269.
Drake, C.J. 1914. The food of Rana pipiens Schreber. Ohio Naturalist 14: 257-269. Dunlap, D.G. 1979. Hemoglobin phenotypes in the frogs Rana pipiens, Rana blairi, and their hybrids and a backcross. Comparative Biochemistry and Physiology B. 62:167-173.
Dunlap, D.G. 1979. Hemoglobin phenotypes in the frogs Rana pipiens, Rana blairi, and their hybrids and a backcross. Comparative Biochemistry and Physiology B. 62:167-173. Dunlap, D.G. 1982. Linkage analysis of the transferrin, albumin, and hemoglobin loci in leopard frogs. Journal of Heredity 73: 247-248.
Dunlap, D.G. 1982. Linkage analysis of the transferrin, albumin, and hemoglobin loci in leopard frogs. Journal of Heredity 73: 247-248. Dunlap, D.G. and K.C. Kruse. 1976. Frogs of the Rana pipiens complex in the northern and central plains states. Southwest Naturalist 20(4): 559-571.
Dunlap, D.G. and K.C. Kruse. 1976. Frogs of the Rana pipiens complex in the northern and central plains states. Southwest Naturalist 20(4): 559-571. Econ, Inc. 1988. Wildlife monitoring report, 1987 field season, Big Sky Mine. March 1988. In Peabody Mining and Reclamation Plan Big Sky Mine Area B. Vol. 8, cont., Tab 10 - Wildlife Resources. Appendix 10-1, 1987 Annual Wildlife Report.
Econ, Inc. 1988. Wildlife monitoring report, 1987 field season, Big Sky Mine. March 1988. In Peabody Mining and Reclamation Plan Big Sky Mine Area B. Vol. 8, cont., Tab 10 - Wildlife Resources. Appendix 10-1, 1987 Annual Wildlife Report. Eddy, S.B. 1976. Population ecology of the leopard frog, Rana pipiens pipiens Schreber, at Delta Marsh, Manitoba. M.S. Thesis, University of Manitoba, 84 pp.
Eddy, S.B. 1976. Population ecology of the leopard frog, Rana pipiens pipiens Schreber, at Delta Marsh, Manitoba. M.S. Thesis, University of Manitoba, 84 pp. Ehrlich, D. 1979. Predation by bullfrog tadpoles (Rana catesbeiana) on eggs and newly hatched larvae of the plains leopard frog (Rana blairi). Bulletin of the Maryland Herpetological Society 15: 25-26.
Ehrlich, D. 1979. Predation by bullfrog tadpoles (Rana catesbeiana) on eggs and newly hatched larvae of the plains leopard frog (Rana blairi). Bulletin of the Maryland Herpetological Society 15: 25-26. Enk, M. 1999. Preliminary results of amphibian monitoring on the Lewis & Clark National Forest. Intermountain Journal of Sciences 5(1-4): 48.
Enk, M. 1999. Preliminary results of amphibian monitoring on the Lewis & Clark National Forest. Intermountain Journal of Sciences 5(1-4): 48. Farmer, P. 1980. Terrestrial wildlife monitoring study, Pearl area, Montana June, 1978 - May, 1980. Western Technology and Engineering, Inc. Helena, MT.
Farmer, P. 1980. Terrestrial wildlife monitoring study, Pearl area, Montana June, 1978 - May, 1980. Western Technology and Engineering, Inc. Helena, MT. Farmer, P. and S.B. Heath. 1987. Wildlife baseline inventory, Rock Creek study area, Sanders County, Montana. Western Technology and Engineering, Inc. Helena, MT.
Farmer, P. and S.B. Heath. 1987. Wildlife baseline inventory, Rock Creek study area, Sanders County, Montana. Western Technology and Engineering, Inc. Helena, MT. Farmer, P. J. 1980. Terrestrial Wildlife Monitoring Study, Pearl Area, Montana, June, 1978 - May, 1980. Tech. Rep. by WESTECH for Shell Oil Co.
Farmer, P. J. 1980. Terrestrial Wildlife Monitoring Study, Pearl Area, Montana, June, 1978 - May, 1980. Tech. Rep. by WESTECH for Shell Oil Co. Feigley, H. P. 1997. Colonial nesting bird survey on the Bureau of Land Management Lewistown District: 1996. Unpublished report, U.S. Bureau of Land Management, Lewistown, Montana.
Feigley, H. P. 1997. Colonial nesting bird survey on the Bureau of Land Management Lewistown District: 1996. Unpublished report, U.S. Bureau of Land Management, Lewistown, Montana. Fernandez, P.J. and J.T. Bagnara. 1995. Recent changes in leopard frog distribution in the White Mountains of east central Arizonia. Declining Amphibian Populations Task Force- Southwestern United States Working Group.
Fernandez, P.J. and J.T. Bagnara. 1995. Recent changes in leopard frog distribution in the White Mountains of east central Arizonia. Declining Amphibian Populations Task Force- Southwestern United States Working Group. Fjell, Alan K., 1986, Peabody Coal Company Big Sky Mine, Rosebud County, MT. Wildlife monitoring report: 1985 field season. March 1986.
Fjell, Alan K., 1986, Peabody Coal Company Big Sky Mine, Rosebud County, MT. Wildlife monitoring report: 1985 field season. March 1986. Fjell, Alan K., and Brian R. Mahan., 1987, Big Sky Mine, Rosebud County, MT. Wildlife monitoring report: 1986 field season. April 1987.
Fjell, Alan K., and Brian R. Mahan., 1987, Big Sky Mine, Rosebud County, MT. Wildlife monitoring report: 1986 field season. April 1987. Flath, D.L. 1998. Species of special interest or concern. Montana Department of Fish, Widlife and Parks, Helena, MT. March, 1998. 7 p.
Flath, D.L. 1998. Species of special interest or concern. Montana Department of Fish, Widlife and Parks, Helena, MT. March, 1998. 7 p. Flath, D.L. 2002. Reptile and amphibian surveys in the Madison-Missouri River Corridor, Montana. Annual Progress Report. 14pp.
Flath, D.L. 2002. Reptile and amphibian surveys in the Madison-Missouri River Corridor, Montana. Annual Progress Report. 14pp. Fogleman, J.C. 1974. The distribution and inheritance of a dorsal color polymorphism in the leopard frog (Rana pipiens). Masters Thesis, Colorado State University.
Fogleman, J.C. 1974. The distribution and inheritance of a dorsal color polymorphism in the leopard frog (Rana pipiens). Masters Thesis, Colorado State University. Fontenot, L.W., G.P. Noblet and S.G. Platt. 1994. Rotenone hazards to amphibians and reptiles. Herpetological Review 25(4):150-156.
Fontenot, L.W., G.P. Noblet and S.G. Platt. 1994. Rotenone hazards to amphibians and reptiles. Herpetological Review 25(4):150-156. Forbes, M.R., D.L. McRuer, and P.L. Rutherford. 2004. Prevalence of Aeromonas hydrophilia in relation to timing and duration of breeding in three species of ranid frogs. Ecoscience 11(3):282-285.
Forbes, M.R., D.L. McRuer, and P.L. Rutherford. 2004. Prevalence of Aeromonas hydrophilia in relation to timing and duration of breeding in three species of ranid frogs. Ecoscience 11(3):282-285. Force, E.R. 1925. Notes on reptiles and amphibians of Okmulgee County, Oklahoma. Copeia 1925(141): 25-27.
Force, E.R. 1925. Notes on reptiles and amphibians of Okmulgee County, Oklahoma. Copeia 1925(141): 25-27. Force, E.R. 1933. The age of attainment of sexual maturity of the leopard frog (Rana pipiens) (Shreber), in northern Michigan. Copeia 1933(3): 128-131.
Force, E.R. 1933. The age of attainment of sexual maturity of the leopard frog (Rana pipiens) (Shreber), in northern Michigan. Copeia 1933(3): 128-131. Franz, R. 1971. Notes on the distribution and ecology of the herpetofauna of northwestern Montana. Bulletin of the Maryland Herpetological Society 7: 1-10.
Franz, R. 1971. Notes on the distribution and ecology of the herpetofauna of northwestern Montana. Bulletin of the Maryland Herpetological Society 7: 1-10. Freda, J. and D.G. McDonald. 1990. The effects of aluminum on the leopard frog (Rana pipiens): life stage comparions and aluminum uptake. Canadian Journal of Fish and Aquatic Science 47217-224.
Freda, J. and D.G. McDonald. 1990. The effects of aluminum on the leopard frog (Rana pipiens): life stage comparions and aluminum uptake. Canadian Journal of Fish and Aquatic Science 47217-224. Freda, J. and W.A. Dunson. 1984. Sodium balance of amphibian larvae exposed to low environmental pH. Physiological Zoology 57(4): 435-443.
Freda, J. and W.A. Dunson. 1984. Sodium balance of amphibian larvae exposed to low environmental pH. Physiological Zoology 57(4): 435-443. Frost, J.S. 1982. Functional genetic similarity between geographically separated populations of Mexican leopard frogs (Rana pipiens complex). Systematic Zoology 31: 57-67.
Frost, J.S. 1982. Functional genetic similarity between geographically separated populations of Mexican leopard frogs (Rana pipiens complex). Systematic Zoology 31: 57-67. Frost, J.S. 1983. Comparative feeding and breeding strategies of a sympatric pair of leopard frpgs (Rana pipiens complex). Journal of Experimental Zoology 225: 135-140.
Frost, J.S. 1983. Comparative feeding and breeding strategies of a sympatric pair of leopard frpgs (Rana pipiens complex). Journal of Experimental Zoology 225: 135-140. Frost, J.S. and J.E. Platz. 1983. Comparative assessment of modes of reproductive isolation among four species of leopard frogs (Rana pipiens complex). Evolution 37: 66-78.
Frost, J.S. and J.E. Platz. 1983. Comparative assessment of modes of reproductive isolation among four species of leopard frogs (Rana pipiens complex). Evolution 37: 66-78. Frost, J.S. and J.T. Bagnara. 1976. A new species of leopard frog (Rana pipiens complex) from northwestern Mexico. Copeia 1976: 332-38.
Frost, J.S. and J.T. Bagnara. 1976. A new species of leopard frog (Rana pipiens complex) from northwestern Mexico. Copeia 1976: 332-38. Frost, J.S. and J.T. Bagnara. 1977. Sympatry between Rana blairi and the southern from of the leopard frog in southeastern Arizona. (Anura:Ranidae). Southwestern Naturalist 22: 443-453.
Frost, J.S. and J.T. Bagnara. 1977. Sympatry between Rana blairi and the southern from of the leopard frog in southeastern Arizona. (Anura:Ranidae). Southwestern Naturalist 22: 443-453. Gaizick, L., G. Gupta, and E. Bass. 2001. Toxicity of Chlorypyrifos to Rana pipiens Embryos. Bulletin of Environmental Contamination and Toxicology 66(3): 386.
Gaizick, L., G. Gupta, and E. Bass. 2001. Toxicity of Chlorypyrifos to Rana pipiens Embryos. Bulletin of Environmental Contamination and Toxicology 66(3): 386. Gallant, N. and K. Teather. 2001. Differences in size, pigmentation, and fluctuating asymmetry in stressed and nonstressed northern leopard frogs (Rana pipiens). Ecoscience 8(4): 430-436.
Gallant, N. and K. Teather. 2001. Differences in size, pigmentation, and fluctuating asymmetry in stressed and nonstressed northern leopard frogs (Rana pipiens). Ecoscience 8(4): 430-436. Garber, C. S. 1992. A survey for spotted frogs (Rana pretiosa), wood frogs (Rana sylvatica), and boreal toads (Bufo boreas) in Wyoming. Wyoming Natural Diversity Database, Laramie. 15 pp. + appendix.
Garber, C. S. 1992. A survey for spotted frogs (Rana pretiosa), wood frogs (Rana sylvatica), and boreal toads (Bufo boreas) in Wyoming. Wyoming Natural Diversity Database, Laramie. 15 pp. + appendix. Garber, C.S. 1995a. A survey for U.S. Forest Service listed "Sensitive" amphibians including the spotted frog (Rana pretiosa), leopard frog (Rana pipiens), tiger salamander (Ambystoma tigrinum) and the boreal toad (Bufo boreas) on the north half of the
Garber, C.S. 1995a. A survey for U.S. Forest Service listed "Sensitive" amphibians including the spotted frog (Rana pretiosa), leopard frog (Rana pipiens), tiger salamander (Ambystoma tigrinum) and the boreal toad (Bufo boreas) on the north half of the Garber, C.S. 1995b. Addendum Number 1 to "A status survey for spotted frogs (Rana pretiosa) wood frogs (Rana sylvatica) and boreal toads (Bufo boreas) in the mountains of southern and eastern Wyoming. Unpublished report prepared by the Wyoming Natural
Garber, C.S. 1995b. Addendum Number 1 to "A status survey for spotted frogs (Rana pretiosa) wood frogs (Rana sylvatica) and boreal toads (Bufo boreas) in the mountains of southern and eastern Wyoming. Unpublished report prepared by the Wyoming Natural Gates, M.T. 2005. Amphibian and reptile baseline survey: CX field study area Bighorn County, Montana. Report to Billings and Miles City Field Offices of Bureau of Land Management. Maxim Technologies, Billings, MT. 28pp + Appendices.
Gates, M.T. 2005. Amphibian and reptile baseline survey: CX field study area Bighorn County, Montana. Report to Billings and Miles City Field Offices of Bureau of Land Management. Maxim Technologies, Billings, MT. 28pp + Appendices. Gendron, A.D., D.J. Marcogliese, S. Barbeau, M.-S Christin, P. Brousseau, S. Ruby, D. Cyr, and M. Fournier. 2003. Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia 135(3): 469-476.
Gendron, A.D., D.J. Marcogliese, S. Barbeau, M.-S Christin, P. Brousseau, S. Ruby, D. Cyr, and M. Fournier. 2003. Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia 135(3): 469-476. Gibbs, E.L. 1963. An effective treatment for redleg disease in Rana pipiens. Laboratory Animal Care 13: 781-783.
Gibbs, E.L. 1963. An effective treatment for redleg disease in Rana pipiens. Laboratory Animal Care 13: 781-783. Gibbs, E.L. 1973. Rana pipiens: Health and disease-how little we know. American Zoologist 13: 93-96.
Gibbs, E.L. 1973. Rana pipiens: Health and disease-how little we know. American Zoologist 13: 93-96. Gilbertson, M.K., G.D. Haffner, K.G. Drouillard, A. Albert, and B. Dixon. 2003. Immunosuppression in the northern leopard frog (Rana pipiens) induced by pesticide exposure. Environmental Toxicology and Chemistry 22(1):101-110.
Gilbertson, M.K., G.D. Haffner, K.G. Drouillard, A. Albert, and B. Dixon. 2003. Immunosuppression in the northern leopard frog (Rana pipiens) induced by pesticide exposure. Environmental Toxicology and Chemistry 22(1):101-110. Gilliespie, H. and J.W. Crenshaw. 1966. Hemoglobin variation in Rana pipiens (Amphibia:Anura). Copeia 1966: 889-893.
Gilliespie, H. and J.W. Crenshaw. 1966. Hemoglobin variation in Rana pipiens (Amphibia:Anura). Copeia 1966: 889-893. Gillis, J. E. 1975. Characterization of a hybridizing complex of leopard frogs. Ph.D. dissertation, Colorado State University, Fort Collins CO xi + 125 pp.
Gillis, J. E. 1975. Characterization of a hybridizing complex of leopard frogs. Ph.D. dissertation, Colorado State University, Fort Collins CO xi + 125 pp. Gillis, J. E. 1979. Adaptive differences in the water economics of two species of leopard frogs from eastern Colorado. Journal of Herpetology 13: 445-450.
Gillis, J. E. 1979. Adaptive differences in the water economics of two species of leopard frogs from eastern Colorado. Journal of Herpetology 13: 445-450. Glennemeier, K.A. amd R.J. Denver. 2001. Sublethal effects of chronic exposure to an organochlorine compound on northern leopard frog (Rana pipiens) tadpoles. Environmental Toxicology 16(4): 287-297.
Glennemeier, K.A. amd R.J. Denver. 2001. Sublethal effects of chronic exposure to an organochlorine compound on northern leopard frog (Rana pipiens) tadpoles. Environmental Toxicology 16(4): 287-297. Glennemeier, K.A. and R.J. Denver. 2002a. Role for corticoids in mediating the reponse of Rana pipiens tadpoles to intraspecific competition. Journal of Experimental Zoology 292(1): 32-40.
Glennemeier, K.A. and R.J. Denver. 2002a. Role for corticoids in mediating the reponse of Rana pipiens tadpoles to intraspecific competition. Journal of Experimental Zoology 292(1): 32-40. Glennemeier, K.A. and R.J. Denver. 2002b. Small changes in whole-body corticosterone content affect larval Rana pipiens fitness components. General and Comparative Endocrinology 127(1):16-25.
Glennemeier, K.A. and R.J. Denver. 2002b. Small changes in whole-body corticosterone content affect larval Rana pipiens fitness components. General and Comparative Endocrinology 127(1):16-25. Golden, D.R., G.R. Smith, and J.E. Rettig. 2001. Effects of age and group size on habitat selection and activity level in Rana pipiens tadpoles. Herpetological Journal 11(2): 69-74.
Golden, D.R., G.R. Smith, and J.E. Rettig. 2001. Effects of age and group size on habitat selection and activity level in Rana pipiens tadpoles. Herpetological Journal 11(2): 69-74. Goraya, J., Y. Wang and J.M. Conlon. 2000. Peptides with antimicrobial activity from four different familes isolated from the skins of the North American frogs Rana luteiventris, Rana berlandie and Rana pipiens. European Journal of Biochemistry 267(3)
Goraya, J., Y. Wang and J.M. Conlon. 2000. Peptides with antimicrobial activity from four different familes isolated from the skins of the North American frogs Rana luteiventris, Rana berlandie and Rana pipiens. European Journal of Biochemistry 267(3) Greding, E.J. 1972. Call specificity and hybrid compatability between Rana pipiens and three other species Rana species in Central America. Copeia 1972: 383-385.
Greding, E.J. 1972. Call specificity and hybrid compatability between Rana pipiens and three other species Rana species in Central America. Copeia 1972: 383-385. Green, D.M. 1978. Northern leopard frogs and bullfrogs on Vancouver Island. Canadian Field Naturalist 92: 78-79.
Green, D.M. 1978. Northern leopard frogs and bullfrogs on Vancouver Island. Canadian Field Naturalist 92: 78-79. Gromko, H.M. F.S. Mason, and S.J. Smith-Gill. 1973. Analysis of the crowding effect in Rana pipiens tadpoles. Journal of Experimental Zoology 186: 63-72.
Gromko, H.M. F.S. Mason, and S.J. Smith-Gill. 1973. Analysis of the crowding effect in Rana pipiens tadpoles. Journal of Experimental Zoology 186: 63-72. Hanauska-Brown, L., B.A. Maxell, A. Petersen, and S. Story. 2014. Diversity Monitoring in Montana 2008-2010 Final Report. Montana Fish, Wildlife & Parks. Helena, MT. 78 pp.
Hanauska-Brown, L., B.A. Maxell, A. Petersen, and S. Story. 2014. Diversity Monitoring in Montana 2008-2010 Final Report. Montana Fish, Wildlife & Parks. Helena, MT. 78 pp. Hanson, J. No Date. Unpublished report on Youngs Creek. U.S. Fish and Wildlife Service. Hardin, MT. 14 p.
Hanson, J. No Date. Unpublished report on Youngs Creek. U.S. Fish and Wildlife Service. Hardin, MT. 14 p. Hardy, J.D. Jr., and J.H. Gillllepsie. 1976. Hybridization between Rana pipiens and Rana palustris in a modified natural enviroment. Bulletin of the Midland Herpetelogical Society 12: 41-53.
Hardy, J.D. Jr., and J.H. Gillllepsie. 1976. Hybridization between Rana pipiens and Rana palustris in a modified natural enviroment. Bulletin of the Midland Herpetelogical Society 12: 41-53. Harris, M.L., L. Chora and J.P. Bogart. 2000. Species- and age-related differences in susceptibility to pesticide exposure for two amphibians, Rana pipiens, and Bufo americanus. Bulletin of Environmental Contamination and Toxicology 64(2): 263-270.
Harris, M.L., L. Chora and J.P. Bogart. 2000. Species- and age-related differences in susceptibility to pesticide exposure for two amphibians, Rana pipiens, and Bufo americanus. Bulletin of Environmental Contamination and Toxicology 64(2): 263-270. Hayden, F.V. 1862. On the geology and natural history of the upper Missouri. Transactions of the American Philosophical Society New Series 12(1): 1-218
Hayden, F.V. 1862. On the geology and natural history of the upper Missouri. Transactions of the American Philosophical Society New Series 12(1): 1-218 Hayes, T., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2002. Feminization of male frogs in the wild. Nature 419: 895-896.
Hayes, T., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2002. Feminization of male frogs in the wild. Nature 419: 895-896. Hayes, T.B., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2003. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environmnetal Health Perspectives 111(4): 568-575.
Hayes, T.B., K. Haston, M. Tsui, A. Hoang, C. Haeffele, and A. Vonk. 2003. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environmnetal Health Perspectives 111(4): 568-575. Heinen, J.T. and G. Hammond. 1997. Antipredator behaviors of newly metamorphosed green frogs (Rana clamitans) and leopard frogs (R. pipiens) in encounters with eastern garter snakes (Thamnophis s. sirtalis). American Midland Naturalist 137(1): 136-144.
Heinen, J.T. and G. Hammond. 1997. Antipredator behaviors of newly metamorphosed green frogs (Rana clamitans) and leopard frogs (R. pipiens) in encounters with eastern garter snakes (Thamnophis s. sirtalis). American Midland Naturalist 137(1): 136-144. Hendricks, F.S. 1973. Intestinal contents of Rana pipiens Schreber (Ranidae) larvae. Southwestern Naturalist 18: 99-101.
Hendricks, F.S. 1973. Intestinal contents of Rana pipiens Schreber (Ranidae) larvae. Southwestern Naturalist 18: 99-101. Hendricks, P. 1999a. Amphibian and reptile survey of the Bureau of Land Management Miles City District, Montana. Montana Natural Heritage Program, Helena, MT. 80 p.
Hendricks, P. 1999a. Amphibian and reptile survey of the Bureau of Land Management Miles City District, Montana. Montana Natural Heritage Program, Helena, MT. 80 p. Hendricks, P. 2000. Amphibian and reptile survey of the Thompson Chain of Lakes. A report to the Montana Department of Fish, Wildlife, and Parks. Montana Natural Heritage Program, Helena, MT. 15 p.
Hendricks, P. 2000. Amphibian and reptile survey of the Thompson Chain of Lakes. A report to the Montana Department of Fish, Wildlife, and Parks. Montana Natural Heritage Program, Helena, MT. 15 p. Hendricks, P. and J.D. Reichel. 1998. Amphibian and reptile survey on Montana refuges: 1996. Montana Natural Heritage Program, Helena, MT. 19 p.
Hendricks, P. and J.D. Reichel. 1998. Amphibian and reptile survey on Montana refuges: 1996. Montana Natural Heritage Program, Helena, MT. 19 p. Hendricks, P., S. Lenard, D.M. Stagliano, and B.A. Maxell. 2013. Baseline nongame wildlife surveys on the Fort Peck Indian Reservation. Report to the Assiniboine and Sioux Tribes of the Fort Peck Indian Reservation. Montana Natural Heritage Program, Helena, MT. 83 p.
Hendricks, P., S. Lenard, D.M. Stagliano, and B.A. Maxell. 2013. Baseline nongame wildlife surveys on the Fort Peck Indian Reservation. Report to the Assiniboine and Sioux Tribes of the Fort Peck Indian Reservation. Montana Natural Heritage Program, Helena, MT. 83 p. Hillis, D.M. 1981. Premating isolating mechanisms among three species of the Rana pipiens complex in Texas and southern Oklahoma. Copeia 1981: 312-319.
Hillis, D.M. 1981. Premating isolating mechanisms among three species of the Rana pipiens complex in Texas and southern Oklahoma. Copeia 1981: 312-319. Hillis, D.M. and S. Davis. 1986. Evolution of ribosomal DNA: Fifty million years of recorded history in the frog genus Rana. Evolution 40: 1275-1288.
Hillis, D.M. and S. Davis. 1986. Evolution of ribosomal DNA: Fifty million years of recorded history in the frog genus Rana. Evolution 40: 1275-1288. Hillis, D.M., J.S. Frost, and D.A. Wright. 1983. Phylogeny and biogeography of the Rana pipiens complex: a biochemical evaluation. Systematic Zoology 32(2): 132-143.
Hillis, D.M., J.S. Frost, and D.A. Wright. 1983. Phylogeny and biogeography of the Rana pipiens complex: a biochemical evaluation. Systematic Zoology 32(2): 132-143. Hine, R.L., B.L. Les, B.F. Hellmich and R.C. Vogt. 1981. Leopard frog populations and mortality in Wisconsin, 1974-76. Wisconsin Dept. of Natural Resources Technical Bulletin No. 122. Madison, WI. 39pp.
Hine, R.L., B.L. Les, B.F. Hellmich and R.C. Vogt. 1981. Leopard frog populations and mortality in Wisconsin, 1974-76. Wisconsin Dept. of Natural Resources Technical Bulletin No. 122. Madison, WI. 39pp. Hird, D.W., S.L. Diesch, R.G. McKinnell, E. Gorham, F.B. Martin, S.W. Kurtz and C. Dubrovolny. 1981. Aeromonas hydrophila in wild-caught frogs and tadpoles (Rana pipiens) in Minnesota. Laboratory Animal Science 31(2): 166-169.
Hird, D.W., S.L. Diesch, R.G. McKinnell, E. Gorham, F.B. Martin, S.W. Kurtz and C. Dubrovolny. 1981. Aeromonas hydrophila in wild-caught frogs and tadpoles (Rana pipiens) in Minnesota. Laboratory Animal Science 31(2): 166-169. Hlynka, L.J. 1970. Helminths in Rana pipiens Schreber, and Bufo hemiophrys Cope, from the Delta Marshes, Manitoba. M.S. Thesis, University of Manitoba. 110 pp.
Hlynka, L.J. 1970. Helminths in Rana pipiens Schreber, and Bufo hemiophrys Cope, from the Delta Marshes, Manitoba. M.S. Thesis, University of Manitoba. 110 pp. Hoffman, E.A. and M.S. Blouin. 2004. Evolutionary history of the northern leopard frog: reconstruction of phylogeny, phylogeography, and historical changes in population demography from mitochondrial DNA. Evolution 58(1):145-159.
Hoffman, E.A. and M.S. Blouin. 2004. Evolutionary history of the northern leopard frog: reconstruction of phylogeny, phylogeography, and historical changes in population demography from mitochondrial DNA. Evolution 58(1):145-159. Hoffman, E.A., F.W. Schueler, and M.S. Blouin. 2004. Effective population sizes and temporal stability of genetic structure in Rana pipiens, the northern leopard frog. Evolution 58(11):2536-2537.
Hoffman, E.A., F.W. Schueler, and M.S. Blouin. 2004. Effective population sizes and temporal stability of genetic structure in Rana pipiens, the northern leopard frog. Evolution 58(11):2536-2537. Hoffman, E.A., W.R. Ardren, and M.S. Blouin. 2003. Nine polymorphic microsatellite loci for the northern leopard frog (Rana pipiens). Molecular Ecology Notes 3(1): 115-116.
Hoffman, E.A., W.R. Ardren, and M.S. Blouin. 2003. Nine polymorphic microsatellite loci for the northern leopard frog (Rana pipiens). Molecular Ecology Notes 3(1): 115-116. Hofman, D.E. 1991. 1990 central region leopard frog inventory. Unpubl. Rept., Alberta Fish and Wildife Division, Red Deer, AB. 8pp.
Hofman, D.E. 1991. 1990 central region leopard frog inventory. Unpubl. Rept., Alberta Fish and Wildife Division, Red Deer, AB. 8pp. Hofman, D.E. 1992. 1992 leopard frog monitoring project, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 12 pp.
Hofman, D.E. 1992. 1992 leopard frog monitoring project, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 12 pp. Hofman, D.E. 1994a. 1994 northern leopard frog census, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 6 pp.
Hofman, D.E. 1994a. 1994 northern leopard frog census, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 6 pp. Hofman, D.E. 1994b. 1993 leopard frog monitoring project, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 7 pp.
Hofman, D.E. 1994b. 1993 leopard frog monitoring project, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 7 pp. Hofman, D.E. 1995. 1995 northern leopard frog census, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 6 pp.
Hofman, D.E. 1995. 1995 northern leopard frog census, Princes Spring, Alberta. Unpubl. Rept., Alberta Fish and Wildlife Division, Red Deer, AB. 6 pp. Hoppe, D.M. and R.G. McKinnell. 1994. Pattern polymorphism in leopard frogs of Minnesota and contiguous states. North Dakota Academy of Sciences Proceedings 48: 23.
Hoppe, D.M. and R.G. McKinnell. 1994. Pattern polymorphism in leopard frogs of Minnesota and contiguous states. North Dakota Academy of Sciences Proceedings 48: 23. Hossack, B. and P.S. Corn. 2001. Amphibian survey of Medicine Lake National Wildlife Refuge Complex: 2001. USGS Northern Rocky Mountain Science Center, Aldo Leopold Wilderness Research Institute, Missoula, MT. 13 p.
Hossack, B. and P.S. Corn. 2001. Amphibian survey of Medicine Lake National Wildlife Refuge Complex: 2001. USGS Northern Rocky Mountain Science Center, Aldo Leopold Wilderness Research Institute, Missoula, MT. 13 p. Humphris, Michael., 1993, Wildlife Monitoring Report. Spring Creek Coal Company 1993 Mining Annual Report. Appendix I. April 11, 1993.
Humphris, Michael., 1993, Wildlife Monitoring Report. Spring Creek Coal Company 1993 Mining Annual Report. Appendix I. April 11, 1993. Humphris, Michael., 1994, Wildlife Monitoring Report. Spring Creek Coal Company 1994 Mining Annual Report. Appendix I. April 1994.
Humphris, Michael., 1994, Wildlife Monitoring Report. Spring Creek Coal Company 1994 Mining Annual Report. Appendix I. April 1994. Hunter, B.R., D.L. Carlson, E.D. Seppanen, P.S. Killian, B.K. McKinnell, and R.G. McKinnell. 1989. Are renal carcinomas increasing in Rana pipiens after a decade of reduced prevalence. American Midland Naturalist 122: 307-312.
Hunter, B.R., D.L. Carlson, E.D. Seppanen, P.S. Killian, B.K. McKinnell, and R.G. McKinnell. 1989. Are renal carcinomas increasing in Rana pipiens after a decade of reduced prevalence. American Midland Naturalist 122: 307-312. Hupf, T.H. 1977. Natural histories of two species of leopard frogs, Rana blairi and Rana pipiens, in a zone of sympatry in northeastern Nebraska. M.S. Thesis, University of Nebraska, Lincoln, Nebraska. 125 pp.
Hupf, T.H. 1977. Natural histories of two species of leopard frogs, Rana blairi and Rana pipiens, in a zone of sympatry in northeastern Nebraska. M.S. Thesis, University of Nebraska, Lincoln, Nebraska. 125 pp. Hutchinson, V.H. and M.J. Dady. 1964. The viability of Rana pipiens and Bufo terrestris submerged at different temperatures. Herpetologica 20: 149-162.
Hutchinson, V.H. and M.J. Dady. 1964. The viability of Rana pipiens and Bufo terrestris submerged at different temperatures. Herpetologica 20: 149-162. Jaeger, J.R., B.R. Riddle, R.D. Jennings, and D.F. Bradford. 2001. Rediscovering Rana onca: Evidence for phylogenetically distinct leopard frogs from the border region of Nevada, Utah, and Arizona. Copeia 2001(2): 339-354.
Jaeger, J.R., B.R. Riddle, R.D. Jennings, and D.F. Bradford. 2001. Rediscovering Rana onca: Evidence for phylogenetically distinct leopard frogs from the border region of Nevada, Utah, and Arizona. Copeia 2001(2): 339-354. Jones, Lawrence L. C., W. P. Leonard and D. H. Olson, eds. 2005. Amphibians of the Pacific Northwest. Seattle Audubon Society: Seattle, WA, 227 pp.
Jones, Lawrence L. C., W. P. Leonard and D. H. Olson, eds. 2005. Amphibians of the Pacific Northwest. Seattle Audubon Society: Seattle, WA, 227 pp. Joslin, Gayle, and Heidi B. Youmans. 1999. Effects of recreation on Rocky Mountain wildlife: a review for Montana. [Montana]: Montana Chapter of the Wildlife Society.
Joslin, Gayle, and Heidi B. Youmans. 1999. Effects of recreation on Rocky Mountain wildlife: a review for Montana. [Montana]: Montana Chapter of the Wildlife Society. Kaplan, H.M., T.J. Arnholt, and J.E. Payne. 1967. Toxicity of lead nitrate solutions for frogs (Rana pipiens). Laboratory Animal Care 17(2): 240-246.
Kaplan, H.M., T.J. Arnholt, and J.E. Payne. 1967. Toxicity of lead nitrate solutions for frogs (Rana pipiens). Laboratory Animal Care 17(2): 240-246. Kauffeld, C.F. 1937. The status of the leopard frogs Rana brachycephala and Rana pipiens. Herpetologica 1: 84-87.
Kauffeld, C.F. 1937. The status of the leopard frogs Rana brachycephala and Rana pipiens. Herpetologica 1: 84-87. Kilby, J.D. 1945. A biological analysis of the food and feeding habits of two frogs, Hyla cinerea and Rana pipiens Sphenocephala. Quarterly Journal of the Florida Academy of Sciences 8: 71-104.
Kilby, J.D. 1945. A biological analysis of the food and feeding habits of two frogs, Hyla cinerea and Rana pipiens Sphenocephala. Quarterly Journal of the Florida Academy of Sciences 8: 71-104. Kimberling, D.N., A.R. Ferreira, S.M. Shuster and P. Keim. 1996. RAPD marker estimation of genetic structure among isolated northern leopard frog populations in the south-western USA. Molecular Ecology 5(4): 521-529.
Kimberling, D.N., A.R. Ferreira, S.M. Shuster and P. Keim. 1996. RAPD marker estimation of genetic structure among isolated northern leopard frog populations in the south-western USA. Molecular Ecology 5(4): 521-529. Kocher, T.D. 1986. Genetic differentiation during speciation in the Rana pipiens and Ambystoma tigrinum species complexes. Ph.D. Dissertation, University of Colorado, Boulder, Colorado.
Kocher, T.D. 1986. Genetic differentiation during speciation in the Rana pipiens and Ambystoma tigrinum species complexes. Ph.D. Dissertation, University of Colorado, Boulder, Colorado. Kocher, T.D. and R.D. Sage. 1986. Further genetic analyses of a hybrid zone between leopard frogs (Rana pipiens complex) in central Texas. Evolution 40: 21-33.
Kocher, T.D. and R.D. Sage. 1986. Further genetic analyses of a hybrid zone between leopard frogs (Rana pipiens complex) in central Texas. Evolution 40: 21-33. Korky, J.K. 1978. Differentiation of the larvae of members of the Rana pipiens complex in Nebraska. Copeia 1978: 455-459.
Korky, J.K. 1978. Differentiation of the larvae of members of the Rana pipiens complex in Nebraska. Copeia 1978: 455-459. Kruse, K.C. 1981. Phonotactic responses of female northern leopard frogs (Rana pipiens) to Rana blairi, a presumed hybrid, and conspecific mating trills. Journal of Herpetology 15: 145-150.
Kruse, K.C. 1981. Phonotactic responses of female northern leopard frogs (Rana pipiens) to Rana blairi, a presumed hybrid, and conspecific mating trills. Journal of Herpetology 15: 145-150. Kruse, K.C. and D.G. Dunlap. 1976. Serum albumins and hybridization in two species of the Rana pipiens complex in the north central United States. Copeia 1976: 394-396.
Kruse, K.C. and D.G. Dunlap. 1976. Serum albumins and hybridization in two species of the Rana pipiens complex in the north central United States. Copeia 1976: 394-396. Lambers, J.H.R., J.S. Clark, and B. Beckage. 2002. Density-dependent mortality and the latitudinal gradient in species diversity. Nature 6890: 732-734.
Lambers, J.H.R., J.S. Clark, and B. Beckage. 2002. Density-dependent mortality and the latitudinal gradient in species diversity. Nature 6890: 732-734. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Beaverhead Gateway, Dillon, Montana. Proj. No. 130091.011. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. I.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Beaverhead Gateway, Dillon, Montana. Proj. No. 130091.011. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. I. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Fourchette Creek Reservoir Complex, Phillips County, Montana. Proj. No. 130091.023. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. I.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Fourchette Creek Reservoir Complex, Phillips County, Montana. Proj. No. 130091.023. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. I. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Johnson - Valier, Valier, Montana. Proj. No. 130091.018. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. I.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Johnson - Valier, Valier, Montana. Proj. No. 130091.018. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. I. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Musgrave Lake, Montana. Proj. No. 130091.019. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Musgrave Lake, Montana. Proj. No. 130091.019. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Fourchette Creek Reservoir Complex, Phillips County, Montana. Proj. No. 130091.023. February 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. I.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Fourchette Creek Reservoir Complex, Phillips County, Montana. Proj. No. 130091.023. February 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. I. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Musgrave Lake, Zurich, Montana. Proj. No. 130091.019. May 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. II.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Musgrave Lake, Zurich, Montana. Proj. No. 130091.019. May 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. II. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Perry Ranch, Glacier Co., Montana. Proj. No. 130091.020. May 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. II.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Perry Ranch, Glacier Co., Montana. Proj. No. 130091.020. May 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. II. Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Ridgeway Wetland Complex: Mitigation Pond #9???, Ekalaka, Montana. Proj. No. 130091.025. February 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. II.
Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2002: Ridgeway Wetland Complex: Mitigation Pond #9???, Ekalaka, Montana. Proj. No. 130091.025. February 2003. In 2002 Wetland Mitigation Monitoring Reports, Vol. II. Larson, K.A. 2004. Advertisement call complexity in Northern Leopard frogs, Rana pipiens. Copeia 3:676-682
Larson, K.A. 2004. Advertisement call complexity in Northern Leopard frogs, Rana pipiens. Copeia 3:676-682 Leclair, R., Jr. and J. Castanet. 1987. A skeletochronological assessment of age and growth in the frog Rana pipiens Schreber (Amphibia, Anura) from Quebec. Copeia 1987: 361-369.
Leclair, R., Jr. and J. Castanet. 1987. A skeletochronological assessment of age and growth in the frog Rana pipiens Schreber (Amphibia, Anura) from Quebec. Copeia 1987: 361-369. Lee, P.A. 1965. Histological and biochemical analysis of the annual cycle of growth and secretion in the oviduct of Rana pipiens. Ph.D. Thesis, University of Michigan.
Lee, P.A. 1965. Histological and biochemical analysis of the annual cycle of growth and secretion in the oviduct of Rana pipiens. Ph.D. Thesis, University of Michigan. Levine, R.P., J.A. Monroy, and E.L. Brainard. 2004. Contribution of eye retraction to swallowing performace in the northern leopard frog, Rana pipiens. Journal of Experimental Biology 207(8):1361-1368.
Levine, R.P., J.A. Monroy, and E.L. Brainard. 2004. Contribution of eye retraction to swallowing performace in the northern leopard frog, Rana pipiens. Journal of Experimental Biology 207(8):1361-1368. Lewis, W.M., G.E. Gunning, E. Lyles, and W.L. Bridges. 1961. Food choice of largemouth bass as a function of availability and vulnerability of food items. Transactions of the American Fisheries Society 90(3): 277-280.
Lewis, W.M., G.E. Gunning, E. Lyles, and W.L. Bridges. 1961. Food choice of largemouth bass as a function of availability and vulnerability of food items. Transactions of the American Fisheries Society 90(3): 277-280. Licht, L.E. 1991. Habitat selection of Rana pipiens and Rana sylvatica during exposure to warm and cold temperatures. American Midland Naturalist 125: 259-268.
Licht, L.E. 1991. Habitat selection of Rana pipiens and Rana sylvatica during exposure to warm and cold temperatures. American Midland Naturalist 125: 259-268. Littlejohn, M.J., and R.S. Oldham. 1968. Rana pipiens complex: mating call structure and taxonomy. Science 162: 1003-1005.
Littlejohn, M.J., and R.S. Oldham. 1968. Rana pipiens complex: mating call structure and taxonomy. Science 162: 1003-1005. Livo, L.J. 1981. Leopard frog (Rana pipiens) reproduction in Boulder County, Colorado. M.A. Thesis, University of Colorado, Denver, Colorada. 94 pp.
Livo, L.J. 1981. Leopard frog (Rana pipiens) reproduction in Boulder County, Colorado. M.A. Thesis, University of Colorado, Denver, Colorada. 94 pp. Lotshaw, D.P. 1977. Temperature adaptation and effects of thermal acclimation in Rana sylvatica and Rana catesbeiana. Comparative Biochemistry and Physiology A 56: 287-294.
Lotshaw, D.P. 1977. Temperature adaptation and effects of thermal acclimation in Rana sylvatica and Rana catesbeiana. Comparative Biochemistry and Physiology A 56: 287-294. Lucke, B. 1934. A neoplastic disease of the frog Rana pipiens. American Journal of Cancer 20: 352-379.
Lucke, B. 1934. A neoplastic disease of the frog Rana pipiens. American Journal of Cancer 20: 352-379. Lynch, C. 2000. North American Amphibian Monitoring Program's Montana frog-call survey: a report on a pilot program in south-central Montana started April 2000 and completed in June 2000. Zoo Montana Conservation Through Education Program, Billings MT. 39 p.
Lynch, C. 2000. North American Amphibian Monitoring Program's Montana frog-call survey: a report on a pilot program in south-central Montana started April 2000 and completed in June 2000. Zoo Montana Conservation Through Education Program, Billings MT. 39 p. Lynch, C. 2001. North American Amphibian Monitoring Program's Montana frog-call survey: report on year two of a program in south-central Montana started April 2001 and completed in June 2001. Zoo Montana Conservation Through Education Program, Billings MT. 12 p.
Lynch, C. 2001. North American Amphibian Monitoring Program's Montana frog-call survey: report on year two of a program in south-central Montana started April 2001 and completed in June 2001. Zoo Montana Conservation Through Education Program, Billings MT. 12 p. Lynch, Catherine. 2000. North American Amphibian Monitoring Program's Montana Frog-call Survey, a report on a pilot program in south-central Montana started April, 2000 and completed in June 2000. 36 pp (unnumbered).
Lynch, Catherine. 2000. North American Amphibian Monitoring Program's Montana Frog-call Survey, a report on a pilot program in south-central Montana started April, 2000 and completed in June 2000. 36 pp (unnumbered). Lynch, J.D. 1978. The distribution of leopard frogs (Rana blairi and Rana pipiens) (Amphibia, Anura, Ranidae) in Nebraska. Journal of Herpetology 12: 157-162.
Lynch, J.D. 1978. The distribution of leopard frogs (Rana blairi and Rana pipiens) (Amphibia, Anura, Ranidae) in Nebraska. Journal of Herpetology 12: 157-162. Manion, J.J. and L. Cory. 1952. Winter kill of Rana pipiens in shallow ponds. Herpetology 33: 430-435.
Manion, J.J. and L. Cory. 1952. Winter kill of Rana pipiens in shallow ponds. Herpetology 33: 430-435. Marnell, L. E. 1997. Herpetofauna of Glacier National Park. Northwestern Naturalist 78:17-33.
Marnell, L. E. 1997. Herpetofauna of Glacier National Park. Northwestern Naturalist 78:17-33. Martin, P.R. 1980a. Terrestrial wildlife habitat inventory in southeastern Montana. Montana Department of Fish, Wildlife and Parks and Bureau of Land Management, Helena MT. 114 p.
Martin, P.R. 1980a. Terrestrial wildlife habitat inventory in southeastern Montana. Montana Department of Fish, Wildlife and Parks and Bureau of Land Management, Helena MT. 114 p. Martin, P.R. 1980b. Terrestrial wildlife inventory in selected coal areas of Montana. Montana Department of Fish, Wildlife and Parks and Bureau of Land Management, Helena, MT. 84 p.
Martin, P.R. 1980b. Terrestrial wildlife inventory in selected coal areas of Montana. Montana Department of Fish, Wildlife and Parks and Bureau of Land Management, Helena, MT. 84 p. Martin, P.R., K. Dubois and H.B. Youmans. 1981. Terrestrial wildlife inventory in selected coal areas, Powder River resources area final report. Montana Department of Fish, Wildlife and Parks and Bureau of Land Management, Helena, MT. No. YA-553-CTO- 24. 288 p.
Martin, P.R., K. Dubois and H.B. Youmans. 1981. Terrestrial wildlife inventory in selected coal areas, Powder River resources area final report. Montana Department of Fish, Wildlife and Parks and Bureau of Land Management, Helena, MT. No. YA-553-CTO- 24. 288 p. Matthews, W.L. 1979. Wibaux-Beach wildlife baseline study - nongame species. Bureau of Land Management, Miles City, MT. 93 p.
Matthews, W.L. 1979. Wibaux-Beach wildlife baseline study - nongame species. Bureau of Land Management, Miles City, MT. 93 p. Matthews, W.L. 1980a. Wibaux-Beach comparison study: Sydney, Glendive and Plevna Study Areas. Bureau of Land Management, Miles City, MT. 50 p.
Matthews, W.L. 1980a. Wibaux-Beach comparison study: Sydney, Glendive and Plevna Study Areas. Bureau of Land Management, Miles City, MT. 50 p. Matthews, W.L. 1980b. Wildlife of Prairie County Terry study area. Bureau of Land Management, Miles City, MT. 52 p.
Matthews, W.L. 1980b. Wildlife of Prairie County Terry study area. Bureau of Land Management, Miles City, MT. 52 p. Matthews, W.L. 1981. Broadus-Pumpkin Creek baseline inventory - wildlife. Bureau of Land Management, Miles City, MT. 83 p.
Matthews, W.L. 1981. Broadus-Pumpkin Creek baseline inventory - wildlife. Bureau of Land Management, Miles City, MT. 83 p. Matthews, W.L. 1982. Bloomfield-North Fork Baseline Inventories - Wildlife. Unpublished report to the Bureau of Land Management, Miles City, Montana. 61 pp.
Matthews, W.L. 1982. Bloomfield-North Fork Baseline Inventories - Wildlife. Unpublished report to the Bureau of Land Management, Miles City, Montana. 61 pp. Maxell, B. A. 2000. Management of Montana's amphibians: a review of factors that may present a risk to population viability and accounts on the identification, distribution, taxonomy, habitat use, natural history, and the status and conservation of individual species. Report to USFS Region 1, Order Number 43-0343-0-0224. University of Montana, Wildlife Biology Program. Missoula, MT. 161 p.
Maxell, B. A. 2000. Management of Montana's amphibians: a review of factors that may present a risk to population viability and accounts on the identification, distribution, taxonomy, habitat use, natural history, and the status and conservation of individual species. Report to USFS Region 1, Order Number 43-0343-0-0224. University of Montana, Wildlife Biology Program. Missoula, MT. 161 p. Maxell, B.A. 2002a. Amphibian and aquatic reptile inventories in watersheds in the South and Middle Forks of the Flathead River drainage that contain lakes being considered for application of piscicides and subsequent stocking of west slope cutthroat trout. Report to the Region 1 Office of the U.S. Forest Service and the Montana Department of Fish, Wildlife, and Parks. Montana Cooperative Wildlife Research Unit and Wildlife Biology Program, University of Montana, Missoula, MT. 62 pp.
Maxell, B.A. 2002a. Amphibian and aquatic reptile inventories in watersheds in the South and Middle Forks of the Flathead River drainage that contain lakes being considered for application of piscicides and subsequent stocking of west slope cutthroat trout. Report to the Region 1 Office of the U.S. Forest Service and the Montana Department of Fish, Wildlife, and Parks. Montana Cooperative Wildlife Research Unit and Wildlife Biology Program, University of Montana, Missoula, MT. 62 pp. Maxell, B.A. 2016. Northern Goshawk surveys on the Beartooth, Ashland, and Sioux Districts of the Custer-Gallatin National Forest: 2012-2014. Montana Natural Heritage Program. Helena, MT. 114pp.
Maxell, B.A. 2016. Northern Goshawk surveys on the Beartooth, Ashland, and Sioux Districts of the Custer-Gallatin National Forest: 2012-2014. Montana Natural Heritage Program. Helena, MT. 114pp. McAlister, W.H. 1962. Variation in Rana pipiens Schreber in Texas. American Midland Naturalist 67: 334-363.
McAlister, W.H. 1962. Variation in Rana pipiens Schreber in Texas. American Midland Naturalist 67: 334-363. McEneaney, T. and J. Jensen. 1974. The reptiles and amphibians of the Charles M. Russell National Wildlife Range - 1974. Charles M. Russell National Wildlife Refuge. Lewistown, MT. 3 p.
McEneaney, T. and J. Jensen. 1974. The reptiles and amphibians of the Charles M. Russell National Wildlife Range - 1974. Charles M. Russell National Wildlife Refuge. Lewistown, MT. 3 p. McKinnell, R.G. 1980. Continued diminished prevalence of the Lucke renal andenccarcinoma in Minnesota leopard frogs. American Mindland Naturalist 104: 402-404.
McKinnell, R.G. 1980. Continued diminished prevalence of the Lucke renal andenccarcinoma in Minnesota leopard frogs. American Mindland Naturalist 104: 402-404. McKinnell, R.G. and V.L. Ellis. 1972. Herpesviruses in tumors of postspawning Rana pipiens. Cancer Research, 32: 1154-1159.
McKinnell, R.G. and V.L. Ellis. 1972. Herpesviruses in tumors of postspawning Rana pipiens. Cancer Research, 32: 1154-1159. McKinnell, R.G., D.M. Hoppe, and B.K. McKinnell. 2005. Monitoring pigment pattern morphs of northern leopard frogs. Pp. 328-337. In: Lannoo, M.J. (ed). Amphibian declines: the conservation status of United States species. University of California Pre
McKinnell, R.G., D.M. Hoppe, and B.K. McKinnell. 2005. Monitoring pigment pattern morphs of northern leopard frogs. Pp. 328-337. In: Lannoo, M.J. (ed). Amphibian declines: the conservation status of United States species. University of California Pre Mecham, J.S. 1968a. Evidence of reproductive isolation between two populations of the frog, Rana pipiens, in Arizona. Southwestern Naturalist 13: 35-44.
Mecham, J.S. 1968a. Evidence of reproductive isolation between two populations of the frog, Rana pipiens, in Arizona. Southwestern Naturalist 13: 35-44. Mecham, J.S. 1968b. Studies on evolutionary effects of isolation in the Rana pipiens complex. Yearbook of the American Philosophy Society 1968: 314-316.
Mecham, J.S. 1968b. Studies on evolutionary effects of isolation in the Rana pipiens complex. Yearbook of the American Philosophy Society 1968: 314-316. Mecham, J.S. 1969. New information from experimental crosses on genetic relationships within the Rana pipiens species group. Journal of Experimental Zoology 170: 169-180.
Mecham, J.S. 1969. New information from experimental crosses on genetic relationships within the Rana pipiens species group. Journal of Experimental Zoology 170: 169-180. Mecham, J.S. 1971. Vocalizations of the leopard frog, Rana pipiens, and three related Mexican species. Copeia 1971: 505-516.
Mecham, J.S. 1971. Vocalizations of the leopard frog, Rana pipiens, and three related Mexican species. Copeia 1971: 505-516. Mecham, J.S., M.J. Littlejohn, R.S. Oldham, L.E. Brown, J.R. Brown. 1973. A new species of leopard frog (Rana pipiens complex) from the plains of the central United States. Occassional Papers of Museum of Texas Technical University 18: 1-11.
Mecham, J.S., M.J. Littlejohn, R.S. Oldham, L.E. Brown, J.R. Brown. 1973. A new species of leopard frog (Rana pipiens complex) from the plains of the central United States. Occassional Papers of Museum of Texas Technical University 18: 1-11. Merrell, D.J. 1965. The distribution of the dominant burnsi gene in the leopard frog. Evolution 19: 69-85.
Merrell, D.J. 1965. The distribution of the dominant burnsi gene in the leopard frog. Evolution 19: 69-85. Merrell, D.J. 1968. A comparison of the estimated size and the "effective size" of breeding populations of the leopard frog (Rana pipiens). Evolution 22: 274-283.
Merrell, D.J. 1968. A comparison of the estimated size and the "effective size" of breeding populations of the leopard frog (Rana pipiens). Evolution 22: 274-283. Merrell, D.J. 1970. Migration and gene dispersal in Rana pipiens. American Zoologist 10: 47-52.
Merrell, D.J. 1970. Migration and gene dispersal in Rana pipiens. American Zoologist 10: 47-52. Merrell, D.J. 1977. Life history of the leopard frog, Rana pipiens, in Minnesota. Bell Museum of Natural History Occasional Paper 15: 23 pp.
Merrell, D.J. 1977. Life history of the leopard frog, Rana pipiens, in Minnesota. Bell Museum of Natural History Occasional Paper 15: 23 pp. Merrell, D.J. and C.F. Rodell. 1968. Seasonal selection in the leopard frog. Evolution 22: 284-288.
Merrell, D.J. and C.F. Rodell. 1968. Seasonal selection in the leopard frog. Evolution 22: 284-288. Meteyer, C.U., I.K. Loeffler, J.G. Burkhart, K.A. Converse, E. Green, J.C. Helgen, S. Kersten, R. Levey, L. Eaton-Poole, and J.F. Fallon. 2000. Hind limb malformations in free-living northern leopard frogs (Rana pipiens) from Maine, Minnesota, and Vermont suggest multiple etiologies. Teratology 60: 151-171.
Meteyer, C.U., I.K. Loeffler, J.G. Burkhart, K.A. Converse, E. Green, J.C. Helgen, S. Kersten, R. Levey, L. Eaton-Poole, and J.F. Fallon. 2000. Hind limb malformations in free-living northern leopard frogs (Rana pipiens) from Maine, Minnesota, and Vermont suggest multiple etiologies. Teratology 60: 151-171. Montana Dept. of Transportation., 200?, Montana Dept. of Transportation Biological Resources Report: Wetland mitigation east of Browning. Montana Wetland ?? Proj. No. NH 0002(232) CN 0703. In Perry Ranch - East of Browning Wetland Mitigation Site, Glacier County. US#3. Man.? Fin Dist3 Admin.Dist3
Montana Dept. of Transportation., 200?, Montana Dept. of Transportation Biological Resources Report: Wetland mitigation east of Browning. Montana Wetland ?? Proj. No. NH 0002(232) CN 0703. In Perry Ranch - East of Browning Wetland Mitigation Site, Glacier County. US#3. Man.? Fin Dist3 Admin.Dist3 Moore, J.A. 1941. Developmental rates of hybrids between Rana pipiens and Rana sphenocephala. Proceedings of the Society of Experimental Biology Med., 47: 207-210.
Moore, J.A. 1941. Developmental rates of hybrids between Rana pipiens and Rana sphenocephala. Proceedings of the Society of Experimental Biology Med., 47: 207-210. Moore, J.A. 1942b. Study of embryonic temperature tolerance and rate of development in Rana pipiens from different lattitudes. Yearbook of the American Philosophy Society, 1942: 175-178.
Moore, J.A. 1942b. Study of embryonic temperature tolerance and rate of development in Rana pipiens from different lattitudes. Yearbook of the American Philosophy Society, 1942: 175-178. Moore, J.A. 1944. Geographic variation in Rana pipiens Schreber of eastern North America. Bulletin of the American Museum of Natural History 82: 345-369.
Moore, J.A. 1944. Geographic variation in Rana pipiens Schreber of eastern North America. Bulletin of the American Museum of Natural History 82: 345-369. Moore, J.A. 1946. Hybridization between Rana palustris and different geographical forms of Rana pipiens. Proceedings of the National Academy of Science USA 32: 209-212.
Moore, J.A. 1946. Hybridization between Rana palustris and different geographical forms of Rana pipiens. Proceedings of the National Academy of Science USA 32: 209-212. Moore, J.A. 1947. Hybridization between Rana pipiens from Vermont and eastern Mexico. Proceedings of the National Academy of Science USA 33: 72-75.
Moore, J.A. 1947. Hybridization between Rana pipiens from Vermont and eastern Mexico. Proceedings of the National Academy of Science USA 33: 72-75. Moore, J.A. 1949. Geographic variation of adaptive characters in Rana pipiens Schreber. Evolution 3(1): 1-24.
Moore, J.A. 1949. Geographic variation of adaptive characters in Rana pipiens Schreber. Evolution 3(1): 1-24. Moore, J.A. 1950. Further studies on Rana pipiens racial hybrids. American Naturalist 84: 247-254.
Moore, J.A. 1950. Further studies on Rana pipiens racial hybrids. American Naturalist 84: 247-254. Moore, J.A. 1975. Rana pipiens – the changing paradigm. American Zoologist 15: 837-849.
Moore, J.A. 1975. Rana pipiens – the changing paradigm. American Zoologist 15: 837-849. Mundinger, J.G. 1975. The influence of rest-rotation grazing management on waterfowl production on stock-water reservoirs in Phillips County, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p.
Mundinger, J.G. 1975. The influence of rest-rotation grazing management on waterfowl production on stock-water reservoirs in Phillips County, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p. Muths, E. 2003. A radio transmitter belt for small Ranid frogs. Herpetological Review 34(4):345-348.
Muths, E. 2003. A radio transmitter belt for small Ranid frogs. Herpetological Review 34(4):345-348. Noble, G.K. and L.R. Aronson. 1942. The sexual behavior of Anura. 1. The normal mating pattern of Rana pipiens. Bulletin American Museum of Natural History 80: 127-142.
Noble, G.K. and L.R. Aronson. 1942. The sexual behavior of Anura. 1. The normal mating pattern of Rana pipiens. Bulletin American Museum of Natural History 80: 127-142. Noland, R. and G.R. Ultsch. 1981. The roles of temperature and dissolved oxygen in microhabitat selection by the tadpole of a frog (Rana pipiens) and a toad (Bufo terrestris). Copeia 1981: 645-652.
Noland, R. and G.R. Ultsch. 1981. The roles of temperature and dissolved oxygen in microhabitat selection by the tadpole of a frog (Rana pipiens) and a toad (Bufo terrestris). Copeia 1981: 645-652. OEA Research. 1985. Wildlife Inventory:Monitoring Report for the CX Ranch Project. 1983-1984. Unpublished report for Consolidation Coal Company, Pittsburgh, Pennsylvania.
OEA Research. 1985. Wildlife Inventory:Monitoring Report for the CX Ranch Project. 1983-1984. Unpublished report for Consolidation Coal Company, Pittsburgh, Pennsylvania. Oechsli, L.M. 2000. Ex-urban development in the Rocky Mountain West: consequences for native vegetation, wildlife diversity, and land-use planning in Big Sky, Montana. M.Sc. Thesis. Montana State University, Bozeman. 73 p.
Oechsli, L.M. 2000. Ex-urban development in the Rocky Mountain West: consequences for native vegetation, wildlife diversity, and land-use planning in Big Sky, Montana. M.Sc. Thesis. Montana State University, Bozeman. 73 p. Oldham, R.S. 1974. Mate attraction by vocalization in members of the Rana pipiens complex. Copeia 1974(4): 982-984.
Oldham, R.S. 1974. Mate attraction by vocalization in members of the Rana pipiens complex. Copeia 1974(4): 982-984. Patla, D.A. and C.R. Peterson. 1998. Amphibians of the Greater Yellowstone Ecosystem. Natural Resource Conservation Cooperative News 11(Autumn 1998): 10-11.
Patla, D.A. and C.R. Peterson. 1998. Amphibians of the Greater Yellowstone Ecosystem. Natural Resource Conservation Cooperative News 11(Autumn 1998): 10-11. Patla, D.A. and C.R. Peterson. 2001. Status and trends of amphibian populations in the Greater Yellowstone Ecosystem, progress report, February 2001. Greater Yellowstone Ecosystem Amphibian Survey and Monitoring Program, Herpetology Laboratory, Department of Biological Sciences, Idaho State University, Pocatello, ID. 8 p.
Patla, D.A. and C.R. Peterson. 2001. Status and trends of amphibian populations in the Greater Yellowstone Ecosystem, progress report, February 2001. Greater Yellowstone Ecosystem Amphibian Survey and Monitoring Program, Herpetology Laboratory, Department of Biological Sciences, Idaho State University, Pocatello, ID. 8 p. Peterson, C.R. and J.P. Shive. 2002. Herpetological survey of southcentral Idaho. Idaho Bureau of Land Management Technical Bulletin 02-3:1-97.
Peterson, C.R. and J.P. Shive. 2002. Herpetological survey of southcentral Idaho. Idaho Bureau of Land Management Technical Bulletin 02-3:1-97. Pilliod, D.S. and T.C. Esque. 2023. Amphibians and reptiles. pp. 861-895. In: L.B. McNew, D.K. Dahlgren, and J.L. Beck (eds). Rangeland wildlife ecology and conservation. Cham, Switzerland: Springer. 1023 p.
Pilliod, D.S. and T.C. Esque. 2023. Amphibians and reptiles. pp. 861-895. In: L.B. McNew, D.K. Dahlgren, and J.L. Beck (eds). Rangeland wildlife ecology and conservation. Cham, Switzerland: Springer. 1023 p. Platz, J.E. 1972. Sympatric interaction between two forms of leopard frogs (Rana pipiens Complex) in Texas. Copeia 1972: 232-240.
Platz, J.E. 1972. Sympatric interaction between two forms of leopard frogs (Rana pipiens Complex) in Texas. Copeia 1972: 232-240. Platz, J.E. 1976. Biochemical and morphological variation of leopard frogs in Arizona. Copeia 1976: 660-672.
Platz, J.E. 1976. Biochemical and morphological variation of leopard frogs in Arizona. Copeia 1976: 660-672. Platz, J.E. and A.L. Platz. 1973. Rana pipiens complex: hemoglobin phenotypes of sympatric and allopatric populations in Arizona. Science 179: 1334-1336.
Platz, J.E. and A.L. Platz. 1973. Rana pipiens complex: hemoglobin phenotypes of sympatric and allopatric populations in Arizona. Science 179: 1334-1336. Pope, S.E., L. Fahrig, and H.G. Merriam. 2000. Landscape complementation and metapopulation effects on leopard frog populations. Ecology 81(9): 2498-2508.
Pope, S.E., L. Fahrig, and H.G. Merriam. 2000. Landscape complementation and metapopulation effects on leopard frog populations. Ecology 81(9): 2498-2508. Porter, W.P. and C.R. Tracy. 1974. Modeling the effects of temperature changes on the ecology of the garter snake and leopard frog. Pages 594-609. In: Gibbons, J.W. and R.R. Sharitz (eds.). Thermal ecology symposium: U.S. Atomic Energy Commission Conference 730505. Government Printing Office, Washington, D.C.
Porter, W.P. and C.R. Tracy. 1974. Modeling the effects of temperature changes on the ecology of the garter snake and leopard frog. Pages 594-609. In: Gibbons, J.W. and R.R. Sharitz (eds.). Thermal ecology symposium: U.S. Atomic Energy Commission Conference 730505. Government Printing Office, Washington, D.C. Post, D.D. 1972. Species differentiation in the Rana pipiens complex. Ph.D. dissertation, Colorado State University, Fort Collins, CO. 137 pp.
Post, D.D. 1972. Species differentiation in the Rana pipiens complex. Ph.D. dissertation, Colorado State University, Fort Collins, CO. 137 pp. Post, D.D. and D. Pettus. 1966. Variation in Rana pipiens (Anura: Ranidae) of eastern Colorado. Southwestern Naturalist 11: 476-482.
Post, D.D. and D. Pettus. 1966. Variation in Rana pipiens (Anura: Ranidae) of eastern Colorado. Southwestern Naturalist 11: 476-482. Post, D.D. and D. Pettus. 1967. Sympatry of two members of the Rana pipiens complex in Colorado. Herpetologica 23: 323.
Post, D.D. and D. Pettus. 1967. Sympatry of two members of the Rana pipiens complex in Colorado. Herpetologica 23: 323. Powder River Eagle Studies, Inc., Gillette, WY., 2000, Spring Creek Mine 1999 Wildlife Monitoring. March 2000.
Powder River Eagle Studies, Inc., Gillette, WY., 2000, Spring Creek Mine 1999 Wildlife Monitoring. March 2000. Powder River Eagle Studies, Inc., Gillette, WY., 2000, Spring Creek Mine 2000 Wildlife Monitoring. March 2000.
Powder River Eagle Studies, Inc., Gillette, WY., 2000, Spring Creek Mine 2000 Wildlife Monitoring. March 2000. Quaye, M.O. 1973. The taxonomy of the lung-worm Rhabdias Stiles and Hassall, (Nematoda), parasitic in Bufo hemiophrys Cope and Rana pipiens Schreber, and the interspecific relationship of helminths in the lungs of these amphibians. M.S. Thesis, Univers
Quaye, M.O. 1973. The taxonomy of the lung-worm Rhabdias Stiles and Hassall, (Nematoda), parasitic in Bufo hemiophrys Cope and Rana pipiens Schreber, and the interspecific relationship of helminths in the lungs of these amphibians. M.S. Thesis, Univers Rauscher, R.L. 1998. Amphibian and reptile survey on selected Montana Bureau of Reclamation impoundments. Montana Department of Fish, Wildlife and Parks, Nongame Program. Bozeman, MT. 24 pp.
Rauscher, R.L. 1998. Amphibian and reptile survey on selected Montana Bureau of Reclamation impoundments. Montana Department of Fish, Wildlife and Parks, Nongame Program. Bozeman, MT. 24 pp. Rauscher, R.L. 2000. Amphibian and reptile survey on selected Montana Bureau of Reclamation impoundments. Unpublished report, Montana Fish, Wildlife & Parks Non-game Program. 12 pp + maps, tables.
Rauscher, R.L. 2000. Amphibian and reptile survey on selected Montana Bureau of Reclamation impoundments. Unpublished report, Montana Fish, Wildlife & Parks Non-game Program. 12 pp + maps, tables. Reichel, J. and D. Flath. 1995. Identification of Montana's amphibians and reptiles. Montana Outdoors 26(3):15-34.
Reichel, J. and D. Flath. 1995. Identification of Montana's amphibians and reptiles. Montana Outdoors 26(3):15-34. Reichel, J.D. 1997a. Amphibian, reptile and northern bog lemming survey on the Rocky Mountain Front: 1996. Montana Natural Heritage Program, Helena, MT. 81 p.
Reichel, J.D. 1997a. Amphibian, reptile and northern bog lemming survey on the Rocky Mountain Front: 1996. Montana Natural Heritage Program, Helena, MT. 81 p. Relyea, R.A. 2005a. The lethal impact of Roundup on aquatic and terrestrial amphibians. Ecological Applications 15(4): 1118-1124.
Relyea, R.A. 2005a. The lethal impact of Roundup on aquatic and terrestrial amphibians. Ecological Applications 15(4): 1118-1124. Relyea, R.A. 2005b. The lethal impacts of Roundup and predatory stress on six species of North American tadpoles. Archives of Environmental Contamination and Toxicology 48:351-357.
Relyea, R.A. 2005b. The lethal impacts of Roundup and predatory stress on six species of North American tadpoles. Archives of Environmental Contamination and Toxicology 48:351-357. Richards, C.M. 1958. The inhibition of growth in crowded Rana pipiens tadpoles. Physiological Zoology 31: 138-151.
Richards, C.M. 1958. The inhibition of growth in crowded Rana pipiens tadpoles. Physiological Zoology 31: 138-151. Rittschoff, D. 1975. Some aspects of the natural history and ecology of the leopard frog, Rana pipiens. Ph.D. dissertation, University of Michigan. 212 pp.
Rittschoff, D. 1975. Some aspects of the natural history and ecology of the leopard frog, Rana pipiens. Ph.D. dissertation, University of Michigan. 212 pp. Romanchuk, K.A. 2003. Magrath northern leopard frog reintroduction project--year 1 progress report. Alberta Species at Risk Report 79:1-20.
Romanchuk, K.A. 2003. Magrath northern leopard frog reintroduction project--year 1 progress report. Alberta Species at Risk Report 79:1-20. Romanchuk, K.A. and R.W. Quinlan. 2006. Magrath northern leopard frog reintroduction project: final report. Alberta Species at Risk Report 104 : 1-33.
Romanchuk, K.A. and R.W. Quinlan. 2006. Magrath northern leopard frog reintroduction project: final report. Alberta Species at Risk Report 104 : 1-33. Rosine, W.N. 1952. Notes on the occurrence of polydactylism in a second species of Amphibia in Muskee Lake, Colorado. Journal of the Colorado-Wyoming Academy of Science 4(4): 100.
Rosine, W.N. 1952. Notes on the occurrence of polydactylism in a second species of Amphibia in Muskee Lake, Colorado. Journal of the Colorado-Wyoming Academy of Science 4(4): 100. Ruibal, R. 1955. A study of altitudinal races in Rana pipiens. Evolution 9: 322-338.
Ruibal, R. 1955. A study of altitudinal races in Rana pipiens. Evolution 9: 322-338. Ruibal, R. 1959. The ecology of a brackish water population of Rana pipiens. Copeia 1959(4): 315-322.
Ruibal, R. 1959. The ecology of a brackish water population of Rana pipiens. Copeia 1959(4): 315-322. Ruibal, R. 1962. The ecology and genetics of a desert population of Rana pipiens. Copeia 1962: 189-195.
Ruibal, R. 1962. The ecology and genetics of a desert population of Rana pipiens. Copeia 1962: 189-195. Sage, R.D. and R.K. Selander. 1979. Hybridization between species of the Rana pipiens Complex in central Texas. Evolution 33: 1069-1088.
Sage, R.D. and R.K. Selander. 1979. Hybridization between species of the Rana pipiens Complex in central Texas. Evolution 33: 1069-1088. Salthe, S.N. 1969. Geographic variation of the lactate dehydrogenases of Rana pipiens and Rana palustris. Biochemistry and Genetics 2: 271-303.
Salthe, S.N. 1969. Geographic variation of the lactate dehydrogenases of Rana pipiens and Rana palustris. Biochemistry and Genetics 2: 271-303. Sanders, O. 1973. A new leopard frog (Rana berlandieri brownorum) from southern Mexico. Journal of Herpetology 7(2): 87-92.
Sanders, O. 1973. A new leopard frog (Rana berlandieri brownorum) from southern Mexico. Journal of Herpetology 7(2): 87-92. Schmidt, R.S. 1968. Chuckle calls of the leopard frog (Rana pipiens). Copeia 1968(3): 561-569.
Schmidt, R.S. 1968. Chuckle calls of the leopard frog (Rana pipiens). Copeia 1968(3): 561-569. Schook, D.M., and T.K. Bollinger. 2005. An apparent decline of Northern Leopard frogs (Rana pipiens) on the Rafferty Dam Mitigation lands near Estevan, SK. Blue Jay 63(3):144-154.
Schook, D.M., and T.K. Bollinger. 2005. An apparent decline of Northern Leopard frogs (Rana pipiens) on the Rafferty Dam Mitigation lands near Estevan, SK. Blue Jay 63(3):144-154. Schoonover, L.J., and W.H. Marshall. 1951. Food habits of the raccoon (Procyon lotor hirtus) in north-central Minnesota. Journal of Mammalogy 32: 422-428.
Schoonover, L.J., and W.H. Marshall. 1951. Food habits of the raccoon (Procyon lotor hirtus) in north-central Minnesota. Journal of Mammalogy 32: 422-428. Schotthoefer, A. M., A.V. Koehler, C.U. Meteyer, and R.A. Cole. 2003. Influence of Ribeiroia ondatrae (Trematoda: Digenea) infection on limb development and survival of northern leopard frogs (Rana pipiens): effects of hoststage and parasite-exposure level. Canadian Journal of Zoology 81(7): 1144-1153.
Schotthoefer, A. M., A.V. Koehler, C.U. Meteyer, and R.A. Cole. 2003. Influence of Ribeiroia ondatrae (Trematoda: Digenea) infection on limb development and survival of northern leopard frogs (Rana pipiens): effects of hoststage and parasite-exposure level. Canadian Journal of Zoology 81(7): 1144-1153. Schreber, H. 1782. Beitrag zur Naturegeschichte der Frosche. Der Naturforscher 18: 182-193.
Schreber, H. 1782. Beitrag zur Naturegeschichte der Frosche. Der Naturforscher 18: 182-193. Schreber. 1782. Naturforscher, Vol. 18, p. 185, pl. 4.
Schreber. 1782. Naturforscher, Vol. 18, p. 185, pl. 4. Schueler, F.W. 1979. Geographic variation in skin pigmentation and dermal glands in the Northern leopard frog, Rana pipiens. Ph.D. Thesis, University of Toronto.
Schueler, F.W. 1979. Geographic variation in skin pigmentation and dermal glands in the Northern leopard frog, Rana pipiens. Ph.D. Thesis, University of Toronto. Schueler, F.W. 1982a. Geographic variation in skin pigmentation and dermal glands in the northern leopard frog, Rana pipiens. National Museum of Canada Publication in Zoology 16: 80 pp.
Schueler, F.W. 1982a. Geographic variation in skin pigmentation and dermal glands in the northern leopard frog, Rana pipiens. National Museum of Canada Publication in Zoology 16: 80 pp. Scott, N.J. Jr, and R.D. Jennings. 1985. The tadpoles of five species of New Mexican leopard frogs. Occassional Paper of the Museum of Southwestern Biology 3: 1-21.
Scott, N.J. Jr, and R.D. Jennings. 1985. The tadpoles of five species of New Mexican leopard frogs. Occassional Paper of the Museum of Southwestern Biology 3: 1-21. Scow, K.L. 1978. Terrestrial wildlife survey, Zortman and Landusky areas, Little Rocky Mountains, Montana. Unpublished report to Zortman & Landusky Mining Companies, Inc.
Scow, K.L. 1978. Terrestrial wildlife survey, Zortman and Landusky areas, Little Rocky Mountains, Montana. Unpublished report to Zortman & Landusky Mining Companies, Inc. Scow, K.L. 1980. Terrestrial wildlife survey American Colloid study area Phillips County, Montana. Western Technology and Engineering, Inc., Helena, MT.
Scow, K.L. 1980. Terrestrial wildlife survey American Colloid study area Phillips County, Montana. Western Technology and Engineering, Inc., Helena, MT. Sebrun, C.N.L. 1992b. Management plan for the northern leopard forg in Alberta. Unpubl. Rept., Alberta Forestry, Lands, and Wildlife, Edmonton, AB. 52 pp.
Sebrun, C.N.L. 1992b. Management plan for the northern leopard forg in Alberta. Unpubl. Rept., Alberta Forestry, Lands, and Wildlife, Edmonton, AB. 52 pp. Seburn, C.N.L. 1992a. Leopard frog project-field report 1991. Unpubl. Rept. For World Wildlife Fund Canada (Prairie for Tomorrow) and Alberta Forestry, Lands and Wildlife, Edmonton, AB. 59+ pp.
Seburn, C.N.L. 1992a. Leopard frog project-field report 1991. Unpubl. Rept. For World Wildlife Fund Canada (Prairie for Tomorrow) and Alberta Forestry, Lands and Wildlife, Edmonton, AB. 59+ pp. Seburn, C.N.L. 1993. Leopard frog project- progress report 1992. Unpubl. Report for Alberta Forestry, Lands and Wildlife, Edmonton, AB. 32 pp.
Seburn, C.N.L. 1993. Leopard frog project- progress report 1992. Unpubl. Report for Alberta Forestry, Lands and Wildlife, Edmonton, AB. 32 pp. Seburn, C.N.L. 1994. Leopard frog project- progress report 1993. Unpubl. Report for Alberta Forestry, Lands and Wildlife, Edmonton, AB. 15 pp.
Seburn, C.N.L. 1994. Leopard frog project- progress report 1993. Unpubl. Report for Alberta Forestry, Lands and Wildlife, Edmonton, AB. 15 pp. Seburn, C.N.L.and D.C. Seburn. 1996. Status report on western populations of the northern leopard frog (Rana pipiens) in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada, Ottawa, ON.
Seburn, C.N.L.and D.C. Seburn. 1996. Status report on western populations of the northern leopard frog (Rana pipiens) in Canada. Prepared for the Committee on the Status of Endangered Wildlife in Canada, Ottawa, ON. Seburn, D. 1994. Public sightings of northern leopard frogs. Alberta Naturalist 24: 3-4.
Seburn, D. 1994. Public sightings of northern leopard frogs. Alberta Naturalist 24: 3-4. Shinn, E.A. and J.W. Dole. 1978. Evidence for a role for olfactory cues in the feeding response of leopard frogs (Rana pipiens). Herpetologica 34(2): 167-172.
Shinn, E.A. and J.W. Dole. 1978. Evidence for a role for olfactory cues in the feeding response of leopard frogs (Rana pipiens). Herpetologica 34(2): 167-172. Shivers, C.A. and J.M. James. 1970. Morphology and histochemistry of the oviduct and egg-jelly layers in the frog, Rana pipiens. Anatomical Record 166: 541-556.
Shivers, C.A. and J.M. James. 1970. Morphology and histochemistry of the oviduct and egg-jelly layers in the frog, Rana pipiens. Anatomical Record 166: 541-556. Shumway, W. 1940. Stages in the development of Rana pipiens. I. External form, Anatomical Record 78: 138-148.
Shumway, W. 1940. Stages in the development of Rana pipiens. I. External form, Anatomical Record 78: 138-148. Simmons, H.M. 2001. Habitat use and movement of Northern Leopard frogs (Rana Pipiens) in Potholes Reservoir, Grant County, Washington. Northwestern Naturalist 82(2):81.
Simmons, H.M. 2001. Habitat use and movement of Northern Leopard frogs (Rana Pipiens) in Potholes Reservoir, Grant County, Washington. Northwestern Naturalist 82(2):81. Simon, M.P., I. Vatnick, H.A. Hopey, K. Butler, C. Korver, C.Hilton, R.S. Weimann, and M.A. Brodkin. 2002. Effects of acid exposure on natural resistance and mortality of adult Rana pipiens. Journal of Herpetology 36(4):697-699.
Simon, M.P., I. Vatnick, H.A. Hopey, K. Butler, C. Korver, C.Hilton, R.S. Weimann, and M.A. Brodkin. 2002. Effects of acid exposure on natural resistance and mortality of adult Rana pipiens. Journal of Herpetology 36(4):697-699. Spring Creek Coal Company., 1992, Wildlife Monitoring Report. Spring Creek Coal Company 1992 Mining Annual Report. Appendix I.
Spring Creek Coal Company., 1992, Wildlife Monitoring Report. Spring Creek Coal Company 1992 Mining Annual Report. Appendix I. Sredl, M.J. and D.L. Waters. 1995. Status of (most of the) leopard frogs in Arizonia. Declining Amphibian Populations Task Force- Southwestern United States Working Group. Abstracts from January 5 & 6, 1995 Annual Meeting, Phoenix, AZ.
Sredl, M.J. and D.L. Waters. 1995. Status of (most of the) leopard frogs in Arizonia. Declining Amphibian Populations Task Force- Southwestern United States Working Group. Abstracts from January 5 & 6, 1995 Annual Meeting, Phoenix, AZ. Sternthal, D.E. 1974. Olfactory and visual cues in the feeding behavior of the leopard frog (rana pipiens). Zeitschrift fur Tierpsychologie 34: 239-246.
Sternthal, D.E. 1974. Olfactory and visual cues in the feeding behavior of the leopard frog (rana pipiens). Zeitschrift fur Tierpsychologie 34: 239-246. Stewart, R.E., S.A. Reese, and G.R. Ultsch. 2004. The physiology of hibernation in Canadian leopard frog (Rana pipiens) and bullfrogs (Rana catesbeiana). Physiological and Biochemical Zoology 77(1):65-73.
Stewart, R.E., S.A. Reese, and G.R. Ultsch. 2004. The physiology of hibernation in Canadian leopard frog (Rana pipiens) and bullfrogs (Rana catesbeiana). Physiological and Biochemical Zoology 77(1):65-73. Stopper, G.F., L. Hecker, R.A. Franssen, and S.K. Sessions. 2002. How trematodes cause limb deformities in amphibians. Journal of Experimental Zoology 294:252-263.
Stopper, G.F., L. Hecker, R.A. Franssen, and S.K. Sessions. 2002. How trematodes cause limb deformities in amphibians. Journal of Experimental Zoology 294:252-263. Taylor, A.C. and J.J. Kollross. 1946. Stages in the development of Rana pipiens larvae. Anatomical Record 94: 7-23.
Taylor, A.C. and J.J. Kollross. 1946. Stages in the development of Rana pipiens larvae. Anatomical Record 94: 7-23. Test, F.C. 1893. Annotated list of reptiles and batrachians collected. In B.W. Evermann. A reconnaisance of the streams and lakes of western Montana and northwestern Wyoming. Bulletin of United States Fish Commission 11(1891): 57-59.
Test, F.C. 1893. Annotated list of reptiles and batrachians collected. In B.W. Evermann. A reconnaisance of the streams and lakes of western Montana and northwestern Wyoming. Bulletin of United States Fish Commission 11(1891): 57-59. Thompson, L.S. 1981. Circle West wildlife monitoring study: Third annual report. Technical report No. 8. Montana Department of Natural Resources and Conservation. Helena, Montana.
Thompson, L.S. 1981. Circle West wildlife monitoring study: Third annual report. Technical report No. 8. Montana Department of Natural Resources and Conservation. Helena, Montana. Thompson, L.S. 1982. Circle West Wildlife Monitoring Study. Fourth annual report. Technical report 10. Montana Department of Natural Resources and Conservation, Helena, Montana.
Thompson, L.S. 1982. Circle West Wildlife Monitoring Study. Fourth annual report. Technical report 10. Montana Department of Natural Resources and Conservation, Helena, Montana. Thompson, Richard W., Western Resource Dev. Corp., Boulder, CO., 1996, Wildlife baseline report for the Montana [Montanore] Project, Lincoln and Sanders counties, Montana. In Application for a Hard Rock Operating Permit and Proposed Plan of Operation, Montanore Project, Lincoln and Sanders Counties, Montana. Vol. 5. Stroiazzo, John. Noranda Minerals Corp., Libby, MT. Revised September 1996.
Thompson, Richard W., Western Resource Dev. Corp., Boulder, CO., 1996, Wildlife baseline report for the Montana [Montanore] Project, Lincoln and Sanders counties, Montana. In Application for a Hard Rock Operating Permit and Proposed Plan of Operation, Montanore Project, Lincoln and Sanders Counties, Montana. Vol. 5. Stroiazzo, John. Noranda Minerals Corp., Libby, MT. Revised September 1996. Timken, R. No Date. Amphibians and reptiles of the Beaverhead National Forest. Western Montana College, Dillon, MT. 16 p.
Timken, R. No Date. Amphibians and reptiles of the Beaverhead National Forest. Western Montana College, Dillon, MT. 16 p. Turner, F.B. 1951. A checklist of the reptiles and amphibians of Yellowstone National Park with incidental notes. Yellowstone Nature Notes 25(3): 25-29.
Turner, F.B. 1951. A checklist of the reptiles and amphibians of Yellowstone National Park with incidental notes. Yellowstone Nature Notes 25(3): 25-29. Turner, F.B. 1955. Reptiles and amphibians of Yellowstone National Park. Yellowstone Interpretive Series No. 5. Yellowstone Library and Museum Association. Yellowstone National Park, WY. 40 p.
Turner, F.B. 1955. Reptiles and amphibians of Yellowstone National Park. Yellowstone Interpretive Series No. 5. Yellowstone Library and Museum Association. Yellowstone National Park, WY. 40 p. Ultsch, G.R., S.A. Reese, and E.R. Stewart. 2004. Physiology of hibernation in Rana pipiens: metabolic rate, critical oxygen tension, and the effects of hypoxia on several plasma variables. Journal of Experimental Zoology 301A(2):169-176.
Ultsch, G.R., S.A. Reese, and E.R. Stewart. 2004. Physiology of hibernation in Rana pipiens: metabolic rate, critical oxygen tension, and the effects of hypoxia on several plasma variables. Journal of Experimental Zoology 301A(2):169-176. Ultsch, G.R., T.E. Graham, and C.E. Crocker. 2000. An aggregation of overwintering leopard frog (Rana pipiens) and common map Turtles (Graptemys geographica) in northern Vermont. Canadian Field Naturalist 114(2): 314-315.
Ultsch, G.R., T.E. Graham, and C.E. Crocker. 2000. An aggregation of overwintering leopard frog (Rana pipiens) and common map Turtles (Graptemys geographica) in northern Vermont. Canadian Field Naturalist 114(2): 314-315. Van Denburgh, J. 1895. Annotated list of reptiles and batrachians. In C.H. Gilbert and B.W. Evermann. A report upon investigations in the Columbia River Basin, with descriptions of four new species of fishes. Bulletin of United States Fish Commission 14(1894): 206-207.
Van Denburgh, J. 1895. Annotated list of reptiles and batrachians. In C.H. Gilbert and B.W. Evermann. A report upon investigations in the Columbia River Basin, with descriptions of four new species of fishes. Bulletin of United States Fish Commission 14(1894): 206-207. Van Kirk, R., L. Benjamin, and D. Patla. 2000. Riparian area assessment and amphibian status in the watersheds of the Greater Yellowstone Ecosystem. Greater Yellowstone Coalition, Bozeman, MT. 102 p.
Van Kirk, R., L. Benjamin, and D. Patla. 2000. Riparian area assessment and amphibian status in the watersheds of the Greater Yellowstone Ecosystem. Greater Yellowstone Coalition, Bozeman, MT. 102 p. Vitt, L.J., J.P. Caldwell, and D.B. Shepard. 2005. Inventory of amphibians and reptiles in the Billings Field Office Region, Montana. Sam Noble Oklahoma Museum of Natural History and Department of Zoology, University of Oklahoma, Norman, OK. 33 pp.
Vitt, L.J., J.P. Caldwell, and D.B. Shepard. 2005. Inventory of amphibians and reptiles in the Billings Field Office Region, Montana. Sam Noble Oklahoma Museum of Natural History and Department of Zoology, University of Oklahoma, Norman, OK. 33 pp. Volpe, E.P. 1954. Hybrid viability between Rana pipiens from Wisconsin and Mexico. Tulane Studies in Zoology 1:111-23.
Volpe, E.P. 1954. Hybrid viability between Rana pipiens from Wisconsin and Mexico. Tulane Studies in Zoology 1:111-23. Volpe, E.P. 1956. Mutant color patterns in leopard frogs: a possible mulitple allelic series.
Volpe, E.P. 1956. Mutant color patterns in leopard frogs: a possible mulitple allelic series. Waage, B.C. 1998. Western Energy Company Rosebud Mine 1997 annual wildlife monitoring report December 1, 1996 to November 30, 1997 survey period. Western Energy Company, Colstrip, MT.
Waage, B.C. 1998. Western Energy Company Rosebud Mine 1997 annual wildlife monitoring report December 1, 1996 to November 30, 1997 survey period. Western Energy Company, Colstrip, MT. Waage, Bruce C., 1996, Western Energy Company Rosebud Mine, Colstrip, Montana: 1995 Annual Wildlife Monitoring Report; December 1, 1994 - November 30, 1995. February 28, 1996.
Waage, Bruce C., 1996, Western Energy Company Rosebud Mine, Colstrip, Montana: 1995 Annual Wildlife Monitoring Report; December 1, 1994 - November 30, 1995. February 28, 1996. Waage, Bruce C., 1998, Western Energy Company Rosebud Mine, Colstrip, Montana: 1997 Annual Wildlife Monitoring Report; December 1, 1996 - November 30, 1997 Survey Period. March 23, 1998.
Waage, Bruce C., 1998, Western Energy Company Rosebud Mine, Colstrip, Montana: 1997 Annual Wildlife Monitoring Report; December 1, 1996 - November 30, 1997 Survey Period. March 23, 1998. Waage, Bruce C., 1999, Western Energy Company Rosebud Mine, Colstrip, Montana: 1998 Annual Wildlife Monitoring Report; December 1, 1997 - November 30, 1998 Survey Period. February 24, 1999.
Waage, Bruce C., 1999, Western Energy Company Rosebud Mine, Colstrip, Montana: 1998 Annual Wildlife Monitoring Report; December 1, 1997 - November 30, 1998 Survey Period. February 24, 1999. Waage, Bruce C., 2000, Western Energy Company Rosebud Mine, Colstrip, Montana: 1999 Annual Wildlife Monitoring Report; December 1, 1998 - November 30, 1999. February 2000.
Waage, Bruce C., 2000, Western Energy Company Rosebud Mine, Colstrip, Montana: 1999 Annual Wildlife Monitoring Report; December 1, 1998 - November 30, 1999. February 2000. Waage, Bruce C., 2001, Western Energy Company Rosebud Mine, Colstrip, Montana: 2000 Annual Wildlife Monitoring Report; December 1, 1999 - November 30, 2000. March 30, 2001.
Waage, Bruce C., 2001, Western Energy Company Rosebud Mine, Colstrip, Montana: 2000 Annual Wildlife Monitoring Report; December 1, 1999 - November 30, 2000. March 30, 2001. Waage, Bruce C., 2002, Western Energy Company Rosebud Mine, Colstrip, Montana. 2001 Annual Wildlife Monitoring Report; December 1, 2000 - November 30, 2001. Febr. 26, 2002.
Waage, Bruce C., 2002, Western Energy Company Rosebud Mine, Colstrip, Montana. 2001 Annual Wildlife Monitoring Report; December 1, 2000 - November 30, 2001. Febr. 26, 2002. Waitz, J.A. 1959. Parasites infesting R. luteiventris and R. pipiens. M.S. Thesis. Moscow, ID: University of Idaho.
Waitz, J.A. 1959. Parasites infesting R. luteiventris and R. pipiens. M.S. Thesis. Moscow, ID: University of Idaho. Waitz, J.A. 1959. Unpublished Master's Thesis on parasites infesting R. luteiventris and R. pipiens. University of Idaho at Moscow.
Waitz, J.A. 1959. Unpublished Master's Thesis on parasites infesting R. luteiventris and R. pipiens. University of Idaho at Moscow. Wassersug, R.J. 1976a. The identification of leopard frog tadpoles. Copeia 1976: 413-414.
Wassersug, R.J. 1976a. The identification of leopard frog tadpoles. Copeia 1976: 413-414. Waye, H.L. 2001. Teflon tubing as radio transmitter belt material for northern leopard frogs (Rana pipiens). Herpetological Review 32(2): 88-89.
Waye, H.L. 2001. Teflon tubing as radio transmitter belt material for northern leopard frogs (Rana pipiens). Herpetological Review 32(2): 88-89. Weed, A.C. 1922. New frogs from Minnesota. Proceedings of the Biological Society of Washington 35: 107-110.
Weed, A.C. 1922. New frogs from Minnesota. Proceedings of the Biological Society of Washington 35: 107-110. Weick, S.E., M.G. Knutson, B.C. Knights, and B.C. Pember. 2005. A comparison of internal and external radio transmitters with northerm leopard frogs (Rana pipiens). Herpetological Review 36(4):415-421.
Weick, S.E., M.G. Knutson, B.C. Knights, and B.C. Pember. 2005. A comparison of internal and external radio transmitters with northerm leopard frogs (Rana pipiens). Herpetological Review 36(4):415-421. Weis, J.S. 1975. The effect of DDT on tail regeneration in Rana pipiens and R. catesbeiana tadpoles. Copeia 1975: 765-767.
Weis, J.S. 1975. The effect of DDT on tail regeneration in Rana pipiens and R. catesbeiana tadpoles. Copeia 1975: 765-767. Weisel, G.F. 1952. Animal names, anatomical terms, and some ethnozoology of the Flathead Indians. Journal of the Washington Academy of Sciences 42(11): 345-355.
Weisel, G.F. 1952. Animal names, anatomical terms, and some ethnozoology of the Flathead Indians. Journal of the Washington Academy of Sciences 42(11): 345-355. Werner, E.E. 1992. Competitive interactions between wood frog and northern leopard frog larvae: the influence of size and activity. Copeia 1992: 26-35.
Werner, E.E. 1992. Competitive interactions between wood frog and northern leopard frog larvae: the influence of size and activity. Copeia 1992: 26-35. Werner, J.K. 2003. Status of the Northern Leopard Frog (Rana Pipiens) in western Montana. Northwestern Naturalist 84:24-30.
Werner, J.K. 2003. Status of the Northern Leopard Frog (Rana Pipiens) in western Montana. Northwestern Naturalist 84:24-30. Werner, J.K., T. Plummer, and J. Weaselhead. 1998b. The status of amphibians on the Flathead Reservation, Montana. Intermountain Journal of Sciences 4(3-4): 88.
Werner, J.K., T. Plummer, and J. Weaselhead. 1998b. The status of amphibians on the Flathead Reservation, Montana. Intermountain Journal of Sciences 4(3-4): 88. Wershler, C.R. 1992. Northern leopard frog monitoring-1991. Unpubl. Rept. By Sweetgrass Consultants Ltd. For WWF Canada (Prairie for Tomorrow) and Alberta Forestry, Lands and Wildlife, Edmonton, AB. 50 pp.
Wershler, C.R. 1992. Northern leopard frog monitoring-1991. Unpubl. Rept. By Sweetgrass Consultants Ltd. For WWF Canada (Prairie for Tomorrow) and Alberta Forestry, Lands and Wildlife, Edmonton, AB. 50 pp. Western Technology & Engineering, Inc. (WESTECH)., 1991, 1991 Bull Mountains Mine No. 1 Terrestrial Wildlife Monitoring Study. In Meridian Minerals Company Bull Mountains Mine No. 1 Permit Application, Musselshell County, Montana. Vol. 7 of 14: Section 26
Western Technology & Engineering, Inc. (WESTECH)., 1991, 1991 Bull Mountains Mine No. 1 Terrestrial Wildlife Monitoring Study. In Meridian Minerals Company Bull Mountains Mine No. 1 Permit Application, Musselshell County, Montana. Vol. 7 of 14: Section 26 Western Technology and Engineering, Inc. (WESTECH)., 1996, Wildlife Monitoring Absaloka Mine Area Annual Report, 1995. Montana SMP 85005. OSMP Montana 0007D. Febr. 23, 1996.
Western Technology and Engineering, Inc. (WESTECH)., 1996, Wildlife Monitoring Absaloka Mine Area Annual Report, 1995. Montana SMP 85005. OSMP Montana 0007D. Febr. 23, 1996. Western Technology and Engineering, Inc. (WESTECH)., 1997, Wildlife Monitoring Absaloka Mine Area Annual Report, 1996. Montana SMP 85005. OSMP Montana 0007D. Mar. 1997.
Western Technology and Engineering, Inc. (WESTECH)., 1997, Wildlife Monitoring Absaloka Mine Area Annual Report, 1996. Montana SMP 85005. OSMP Montana 0007D. Mar. 1997. Western Technology and Engineering, Inc. (WESTECH)., 1999, Wildlife Monitoring Absaloka Mine Area Annual Report, 1998. SMP 85005. OSMP Montana 0007E. April 1999.
Western Technology and Engineering, Inc. (WESTECH)., 1999, Wildlife Monitoring Absaloka Mine Area Annual Report, 1998. SMP 85005. OSMP Montana 0007E. April 1999. Western Technology and Engineering, Inc. (WESTECH)., 2000, Wildlife Monitoring Absaloka Mine Area Annual Report, 1999. Montana SMP 85005. OSMP Montana 0007E. February 2000.
Western Technology and Engineering, Inc. (WESTECH)., 2000, Wildlife Monitoring Absaloka Mine Area Annual Report, 1999. Montana SMP 85005. OSMP Montana 0007E. February 2000. Western Technology and Engineering, Inc. (WESTECH)., 2001, Wildlife Monitoring Absaloka Mine Area Annual Report, 2000. Montana SMP 85005. OSMP Montana 0007E. February 2001.
Western Technology and Engineering, Inc. (WESTECH)., 2001, Wildlife Monitoring Absaloka Mine Area Annual Report, 2000. Montana SMP 85005. OSMP Montana 0007E. February 2001. Westmoreland Resources, Inc., Hardin, MT., 1981, 1981 Wildlife Report. April 1982.
Westmoreland Resources, Inc., Hardin, MT., 1981, 1981 Wildlife Report. April 1982. Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Ridgeway Wetland Complex with Full Sampling of #9 Ekalaka, Montana. Proj. No. 130091.025. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II.
Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Ridgeway Wetland Complex with Full Sampling of #9 Ekalaka, Montana. Proj. No. 130091.025. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II. Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Ryegate Wetland, Ryegate, Montana. Proj. No. 130091.030. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II.
Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Ryegate Wetland, Ryegate, Montana. Proj. No. 130091.030. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II. Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Wigeon Reservoir, Alzada, Montana. Proj. No. 130091.028. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II.
Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Wigeon Reservoir, Alzada, Montana. Proj. No. 130091.028. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II. Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Wyola-Sunlight Ranch Wetland, Wyola, Montana. Proj. No. 130091.035. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II.
Wetlands West, Inc., Bozeman, MT., and Land & Water Consulting, Inc., Missoula, MT., 2002, Montana Dept. of Transportation Wetland Mitigation Monitoring Report, Year 2001: Wyola-Sunlight Ranch Wetland, Wyola, Montana. Proj. No. 130091.035. July 2002. In 2001 Wetland Mitigation Monitoring Reports, Vol. II. Wheeler, G.C. and J. Wheeler. 1966. The amphibians and reptiles of North Dakota. University of North Dakota, Grand Forks, ND. 104 pp.
Wheeler, G.C. and J. Wheeler. 1966. The amphibians and reptiles of North Dakota. University of North Dakota, Grand Forks, ND. 104 pp. Wied, M.P. 1843. Travels in the interior of North America by Maximilian, Prince of Wied. With numerous engravings on wood, and a large map. Translated from the German by H. Evans Lloyd. To accompany the original series of eighty-one elaborately-coloured plates. Size, imperial folio. Ackerman and Co., London, England. 520 p.
Wied, M.P. 1843. Travels in the interior of North America by Maximilian, Prince of Wied. With numerous engravings on wood, and a large map. Translated from the German by H. Evans Lloyd. To accompany the original series of eighty-one elaborately-coloured plates. Size, imperial folio. Ackerman and Co., London, England. 520 p. Wied, M.P. 1865. Verzeichniss der reptilien, welche auf einer reise in nordlichen America beobachtet wurden, von Maximilian, Prinzen zu Wied. Eigengangen bei der Akademie am 1, Juni 1865. Druck von E. Blochmann und Sohn, Dresden, Germany. 141 p.
Wied, M.P. 1865. Verzeichniss der reptilien, welche auf einer reise in nordlichen America beobachtet wurden, von Maximilian, Prinzen zu Wied. Eigengangen bei der Akademie am 1, Juni 1865. Druck von E. Blochmann und Sohn, Dresden, Germany. 141 p. Wojtaszek, B.F., T.M. Buscarini, D.T. Chartrand, G.R. Stephenson, and D.G. Thompson. 2005. Effect of Release herbicide on mortality, avoidance response, and growth of amphibian larvae in two forest wetlands. Environmental Toxicology and Chemistry 24(10):2533-2544.
Wojtaszek, B.F., T.M. Buscarini, D.T. Chartrand, G.R. Stephenson, and D.G. Thompson. 2005. Effect of Release herbicide on mortality, avoidance response, and growth of amphibian larvae in two forest wetlands. Environmental Toxicology and Chemistry 24(10):2533-2544. Wolff, J.B., M.J. Lee, and C.W. Anderson. 2004. Contribution of the submentalis muscle to feeding mechanics in the leopard frog, Rana pipiens. Journal of Experimental Zoology 301A(8):666-673.
Wolff, J.B., M.J. Lee, and C.W. Anderson. 2004. Contribution of the submentalis muscle to feeding mechanics in the leopard frog, Rana pipiens. Journal of Experimental Zoology 301A(8):666-673. Yaremko, L. 1994. Northern leopard frog project- field report 1994. Unpublished Report For Fish and Wildlife Services, Edmonton, AB. 85pp.
Yaremko, L. 1994. Northern leopard frog project- field report 1994. Unpublished Report For Fish and Wildlife Services, Edmonton, AB. 85pp. Yarrow, H.C. 1882. Check list of North American reptilia and batrachia, with catalogue of specimens in the U.S. National Museum. United States National Museum Bulletin 24. 249 p.
Yarrow, H.C. 1882. Check list of North American reptilia and batrachia, with catalogue of specimens in the U.S. National Museum. United States National Museum Bulletin 24. 249 p. Zenisek, C.J. 1963. A study of the natural history and ecology of the leopard frog, Rana pipiens (Schreber). Ph.D. dissertation, Ohio State University, Columbus, Ohio. 153 pp.
Zenisek, C.J. 1963. A study of the natural history and ecology of the leopard frog, Rana pipiens (Schreber). Ph.D. dissertation, Ohio State University, Columbus, Ohio. 153 pp.
- Web Search Engines for Articles on "Northern Leopard Frog"
- Additional Sources of Information Related to "Amphibians"





