View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Spotted Knapweed - Centaurea stoebe
Other Names:
Centaurea biebersteinii, Centaurea maculosa [misapplied name], Centaurea stoebe ssp. micranthos
State Rank Reason (see State Rank above)
Centaurea stoebe is a plant native to Eurasia and introduced worldwide (FNA 2006). A conservation status rank is not applicable (SNA) because the plant is an exotic (non-native) in Montana that is not a suitable target for conservation activities.
General Description
PLANTS: Taprooted, rosette-forming perennials that grow in terrestrial habitats (Parkinson et al. 2011). Stems are erect, branched, and grow up to 100 cm tall (Lesica 2012). Plants are sparsely gray tomentose and resin-gland-dotted (FNA 2006).
LEAVES: Leaves of the rosette (basal) and lower stem are long-petiolate (Lesica 2012). Leaf blades are ovate, 3–12 cm long, and deeply pinnate (1 to 2 times divided) into linear-oblanceolate lobes.
INFLORESCENCE: Corymbiform with several heads of purplish flowers that occur on the ends of leafy branches (Lesica 2012, Sheley and Petroff 2009). Involucres are ovoid and 8–13 mm high. Involucral bracts (phyllaries) are spine-tipped. Bracts are also marked with fine vertical streaks and tipped with a dark comb-like fringe (Duncan et al. 2011). Inner bracts are lanceolate with swollen tips (Lesica 2012).
Plants in North America are subspecies micranthos (FNA 2006).
Phenology
Flowering June to September, or into October.
Diagnostic Characteristics
The Montana Natural Heritage Botany Program follows the
Centaurea treatment by Keil and Ochsmann in FNA Volume 19 (2006). The
Manual of Montana Vascular Plants (Lesica et al. 2012) treats this plant as
Centaurea maculosa Lam.
For over 200 years there has been a lot of confusion in the European literature regarding the nomenclature used for
Centaurea stoebe (FNA 2006). The names used in this group (
C. stoebe, C. rhenana, C. maculosa, C. biebersteinii) have been applied to different taxa by different authors using different concepts. This was apparent between western and eastern Europe and was not taken into consideration in the treatment by J. Dostal (1976) (FNA 2006).
Recent studies have shown that the American plants are a tetraploid perennial that is very distinct from the diploid, biennial plants native to central Europe (FNA 2006).
Centaurea stoebe ssp.
micranthos being in America while plants in central Europe are known by the names of
C. stoebe Linnaeus ssp.
stoebe,
C. rhenana Boreau, or
C. maculosa Lamarck. In most American literature the name
Centaurea maculosa Lamarck was misapplied to
C. stoebe spp.
micranthos. Others, such as W.A. Weber (1987, 1990) accepted the name of
Acosta maculosa based on a treatment of about 100 plants in the
Centaurea sect.
Acrolophus Cassini (J. Holub et al. 1972). However, the genus
Acosta is not supported by morphologic and molecular characteristics and is not widely accepted in Europe (FNA 2006).
Spotted Knapweed (Centaurea stoebe ssp. micranthos) has unique involucral bracts that have a dark colored tip and fringe that appear as “spots” from a distance.
Diffuse Knapweed (Centaurea diffusa) also grows a single, branched stem from a similar looking rosette. However, its growth develops a ball-shaped appearance and a tumbleweed mobility (Parkinson et al. 2011). Flowers are usually white or occasionally light purple. The flower bracts may have dark-colored tips but lack the dark fringe found in Spotted Knapweed. Bracts possess a rigid terminal spine (1/4 to 1/3 of an inch long) with 4-5 pairs of shorter, lateral spines (Parkinson et al. 2011).
Centaurea stoebe ssp.
micranthos readily hybridizes with
Centaurea diffusa (FNA 2006; Parkinson et al. 2011). These fertile hybrids have been named
Centaurea x
psammogena G. Gayer. Characteristics of the hybrids are variable, except for the cypselae (fruit) which always bears a pappus and the flower heads are always conspicuously radiant (composed of ray florets). Hybrids are often mis-identified as diffuse knapweed and may occur where their parent’s ranges overlap or are separate.
Russian Knapweed (Acroptilon repens) is rhizomatous, whereas, spotted knapweed is taprooted. The involucral bracts are green at the base with papery, translucent tips and are covered with light, thin hairs (Parkinson et al. 2011). The rosette leaves differ in being less dissected and wider (Parkinson et al. 2011).
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Spotted Knapweed is native to southeastern Europe, and introduced to the whole of Europe, northward to Sweden (FNA 2006). It was introduced into North America as a contaminant in alfalfa (Medicago sativa) in Victoria, British Columbia in 1883 (Sheley and Petroff 1999). Other introductions came from discarded soil used as ship ballast (Sheley and Petroff 1999). By 1997 it was found in 14 western states and in every county in Idaho, Montana, Washington, and Wyoming (Sheley and Petroff 1999). If is found in almost every state and several Canadian provinces (FNA 2006).
Occurrences of the hybrid, Centaurea xpsammogena G. Gayer, in Montana are not yet documented.
Adhikari et al. (2020) modeled the predicted future distribution of this and other weed species across the major road network in Montana under predicted future climate scenarios and found that area of predicted future habitat suitability is likely to decrease.
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
Observations in Montana Natural Heritage Program Database
Number of Observations: 154819
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
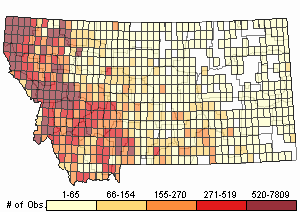
Relative Density
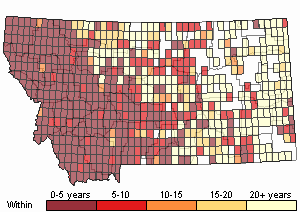
Recency
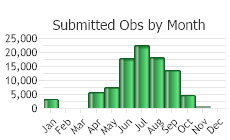
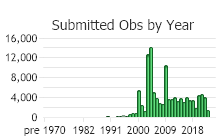
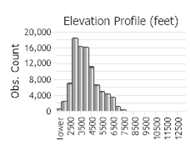 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Grasslands, roadsides, meadows, open forest, and woodlands, particularly where land has been disturbed (Lesica et al. 2012; FNA 2006). Plains, valleys, and montane (Lesica et al. 2012).
Ecology
NUTRIENT VALUEThe rosettes and young flowers of spotted knapweed have some nutritive value. A study by Kelsey and Mihalovich (1987) found that spotted knapweed collected before flowering contained neutral detergent fiber (24.2-53.0% in dry weight), ether extract (3.1-9.0%), crude protein (6.2-18.2%), total non-structural carbohydrates (11.0- 27.5%), ash (4.9 to 9.3%),
in vitro dry matter digestibility (53.2-61.8%), and gross energy of 4,088 to 4,539 calories per gram (Sheley and Petroff 1999). They also found that crude protein and nonstructural carbohydrates were more concentrated in the spring, becoming more fibrous and lower as stems matured (Sheley and Petroff 1999).
POPULATION GROWTHDisturbance facilitates establishment and spread (Sheley and Petroff 1999). Greater densities of spotted knapweed have been correlated to greater levels of soil disturbance (Watson and Renney 1974). Once plants are established, they can develop dense stands because the combination of seedlings, rosettes, and mature plants occupy different rooting zones and all available niches (Sheley and Larson 1996).
Cnicin is an allelopathic compound that has been isolated from spotted knapweed leaves and shoots (Fletcher and Renny 1963). In laboratory experiments cnicin has been shown to reduce the germination of crested wheatgrass (
Agropyron cristatum), bluebunch wheatgrass (
Elymus spicatus), and rough fescue (
Festuca campestris) (Kelsey and Locken 1987). In the field, cnicin was only detected in August at non-toxic levels, and the authors concluded that aggressive resource competition was more important than allelopathy in determining knapweed success (Kelsey and Locken 1987).
In mixed stands the hybrid,
Centaurea x
Psammogena G. Gayer, replaces
Centaurea diffusa (FNA 2006).
POLLINATORS The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus vagans,
Bombus bifarius,
Bombus centralis,
Bombus fervidus,
Bombus rufocinctus,
Bombus ternarius,
Bombus terricola,
Bombus occidentalis,
Bombus griseocollis,
Bombus impatiens,
Bombus insularis, and
Bombus suckleyi (Thorp et al. 1983, Johnson 1986, Colla and Dumesh 2010, Koch et al. 2012, Williams et al. 2014, Tripoldi and Szalanski 2015).
Reproductive Characteristics
FLOWER HEADS
Flower heads consist only of disk flowers (though sometimes they appear radiate) which have both stamens and pistils (are perfect) (Lesica et al. 2012). Each head is composed of 30-40 disk flowers that are purplish-pink, light purple, or sometimes white (Lesica et al. 2012; Parkinson et al. 2011). The pappus consists of white, unequal, stiff bristles in 1 to 2 series (FNA 2006). The outer flowers are enlarged, asymmetrical, and often sterile (Lesica et al. 2012). The inner flowers have corollas of 12–15 mm long. Fruits (cypselae) are whitish or pale brown, 3–4 mm long. and finely hairy (FNA 2006).
SEEDS
Seed germination occurs at temperatures ranging from 45 to 93 degrees Fahrenheit (Sheley and Petroff 1999). Optimal germination occurs at about 66 degrees Fahrenheit under conditions of alternating light and dark environments (Sheley and Petroff 1999). Annual seed production can result in 5,000 to 40,000 seeds per square meter but can be reduced through annual control of spotted knapweed plants (Sheley and Petroff 1999). Seeds ingested by sheep and mule deer can remain viable from zero to three days after travelling through their digestive track (Sheley and Petroff 1999).
LIFE CYCLE
Seeds germinate in the fall and early spring when moisture and temperature are suitable (Sheley and Petroff 1999). Rosettes develop in the spring (Parkinson et al. 2011). During the first year, stems develop from rosettes or the rosette will die back to the root crown and overwinter (Sheley and Petroff 1999). From the rosette, 1-6 stems will bolt in spring to early summer (Sheley and Petroff 1999). Flowering occurs from mid-summer to early fall (Parkinson et al. 2011). Individual flower heads bloom from 2 to 6 days before the bracts close (Parkinson et al. 2011). After about 20 days bracts re-open to disperse seeds (achenes) (Parkinson et al. 2011). Mature seeds are primarily formed by mid-August (Sheley and Petroff 1999). Plants can live up to 9 years, producing seeds each year (Sheley and Petroff 1999).
Management
See
Noxious Weed Education VideoSuccessful management of spotted knapweed requires that land-use objectives and a desired plant community be identified (Sheley and Petroff 1999). Once identified then an integrated weed management strategy that promotes a weed-resistant plant community and serves other land-use objectives such as livestock forage, wildlife habitat, or recreation can be developed, making control of spotted knapweed possible (Sheley and Petroff 1999).
PREVENTION [Adapted from Sheley and Petroff 1999]
Spotted knapweed is spread by wind and movement through established stands. Preventing vehicles from driving through and animals from grazing within infested areas will reduce spread. Hay that is weed-free will reduce spread. Maintaining an intact plant community and reducing soil disturbance will prevent or slow down spread.
BIOCONTROL [Adapted from Jacobs 2007]
At least 200 insects are recommended for establishing a sustainable population. Infestations should be at least 2 acres with sizeable populations. It may take 2-3 years for the insect population to establish. Spotted knapweed is best controlled when at least two insects (specializing in seed heads and roots) are used together. Bio-control insects have been shown in MT to reduce spotted knapweed populations, but control is most effective when used in combination with other tactics. Mouse populations have increased in some areas as Knapweed seed head bio-control species have become their new food source.
In the U.S. 8 flower seedhead and 5 root-boring insect species have been approved for release as bio-control on spotted knapweed.
Knapweed Gall Flies (
Urophora affinis, U. quadrifasciata) were released over 20 years ago in Montana and now are well-established in the western U.S. They have been found to reduce seed production by 50%.
Knapweed Seed Head Weevils (
Larinus minutus, L. obtusus) feed on foliage and flowers and are widely distributed and established.
Knapweed Root Boring Weevil (
Cyphocleonus aschates) is well established and larvae feed on taproots.
Sulphur Knapweed Moth (
Agapeta zoegana) is established in parts of Montana and prefer hot, dry, open sites where larvae attack roots.
Bronze Knapweed Root Borer (
Sphenoptera jugoslavica) is well established in parts of Montana and prefer hot, dry, open sites where larvae attack roots.
CHEMICAL CONTROL [Adapted from Sheley and Petroff 1999; Jacobs 2007]
Herbicides are effective, especially when properly managed with other tactics. The herbicide type and concentration, timing of chemical control, soil properties, and other factors will determine its effectiveness and impact to non-target species. Strict adherence to application requirements defined on the herbicide label will reduce risks to human and environmental health. Many herbicides must be applied by applicators with an Aquatic Pest Control license. Consult your County Extension Agent and/or Weed District for more information on herbicidal control.
Picloram used at 0.25 pound per acre is the standard recommendation and can reduce spotted knapweed populations by 90% on loamy soils with a well-maintained grassland community. It cannot be used in sandy soils, near surface waters, or in areas with a high-water table.
2,4-D is a broadleaf-selective herbicide that works best when used after seeds germinate and before plants develop flowers.
Clopyralid or
triclopyr herbicides do not injure non-target forbs.
Aminopyralid is a more recently developed chemical that has a lower application rate and a shorter soil half-life.
PHYSICAL and CULTURAL CONTROLS [Adapted from Sheley and Petroff 1999; Jacobs 2007]
Hand-pulling that extracts the full taproot is effective, particularly when soil is moist. All plant material should be bagged and desiccated before placing in the trash for disposal. Gloves should be worn to protect skin.
Mowing after plants bolt and before flowering will reduce energy reserves and seed production. Repeated mowing may be necessary. Mowed plants will develop flower heads below the mower blade’s height.
Tilling that severs the taproot near, but below the root crown can reduce populations. However, seed germination may increase until the seed bank is gone.
Burning is not effective, and may stimulate growth or germination.
Revegetation that establishes a desirable perennial plant community will compete against Spotted Knapweed for water, nutrients, and light. Proper revegetation of disturbed sites are necessary to reduce knapweed populations.
GRAZING CONTROLS [Adapted from Sheley and Petroff 1999; Jacobs 2007]
Plants can tolerate defoliation, but severe defoliation will reduce root, crown, and above ground growth. Grazing by cattle is not as effective as by sheep or goat. Cattle prefer grasses over spotted knapweed. Sheep and goats will eat more knapweed, especially when combined with other management tactics. Grazing an area first by cattle and later by sheep, increasing grazing pressures by using electrical fences, or irrigating land followed by repeated sheep grazing are some useful tactics to reduce knapweed. Animals grazing on knapweed should be held for at least 5 days before moving to weed-free areas.
Useful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesStewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Adhikari, A., L.J. Rew, K.P. Mainali, S. Adhikari, and B.D. Maxwell. 2020. Future distribution of invasive weed species across the major road network in the state of Montana, USA. Regional Environmental Change 20(60):1-14. https://doi.org/10.1007/s10113-020-01647-0
Adhikari, A., L.J. Rew, K.P. Mainali, S. Adhikari, and B.D. Maxwell. 2020. Future distribution of invasive weed species across the major road network in the state of Montana, USA. Regional Environmental Change 20(60):1-14. https://doi.org/10.1007/s10113-020-01647-0 Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68. Duncan, C., and J. Story, R. Sheley, H. Parkinson, and J. Mangold. 2011. Biology, ecology and management of Montana knapweeds. Montana State University Extension. Bozeman, MT. 19p.
Duncan, C., and J. Story, R. Sheley, H. Parkinson, and J. Mangold. 2011. Biology, ecology and management of Montana knapweeds. Montana State University Extension. Bozeman, MT. 19p. Fletcher, R. A. And A. J. Renney. 1963. A Growth Inhibitor Found in Centaurea Spp. Can. J. Plant Sci. 43: 475-481.
Fletcher, R. A. And A. J. Renney. 1963. A Growth Inhibitor Found in Centaurea Spp. Can. J. Plant Sci. 43: 475-481. Flora of North America Editorial Committee. 2006. Flora of North America North of Mexico. Vol. 19. Magnoliophyta: Asteridae, part 6: Asteraceae, part 1. Oxford Univ. Press, New York. xxiv + 579 pp.
Flora of North America Editorial Committee. 2006. Flora of North America North of Mexico. Vol. 19. Magnoliophyta: Asteridae, part 6: Asteraceae, part 1. Oxford Univ. Press, New York. xxiv + 579 pp. Jacobs, Jim. 2007. Ecology and Management of Spotted Knapweed (Centaurea maculosa Lam.). July. USDA Natural Resources Conservation Service, Bozeman, Montana.
Jacobs, Jim. 2007. Ecology and Management of Spotted Knapweed (Centaurea maculosa Lam.). July. USDA Natural Resources Conservation Service, Bozeman, Montana. Johnson, R.A. 1986. Intraspecific resource partitioning in the bumble bees Bombus ternarius and B. pennsylvanicus. Ecology 67:133-138.
Johnson, R.A. 1986. Intraspecific resource partitioning in the bumble bees Bombus ternarius and B. pennsylvanicus. Ecology 67:133-138. Kelsey, R.G., and L.J. Locken. 1987. Phytotoxic properties of cnicin, a sequiterpene lactone from Centaurea maculosa (spotted knapweed). J. chem. Ecol. 13(1): 19-33.
Kelsey, R.G., and L.J. Locken. 1987. Phytotoxic properties of cnicin, a sequiterpene lactone from Centaurea maculosa (spotted knapweed). J. chem. Ecol. 13(1): 19-33. Kelsey, R.G., and R.D. Mihalovich. 1987. Nutrient composition of spotted knapweed (Centurea maculosa). J. Range Manage. 40(3):277-281.
Kelsey, R.G., and R.D. Mihalovich. 1987. Nutrient composition of spotted knapweed (Centurea maculosa). J. Range Manage. 40(3):277-281. Koch, J., J. Strange, and P. Williams. 2012. Bumble bees of the western United States. Washington, DC: USDA Forest Service, Pollinator Partnership. 143 p.
Koch, J., J. Strange, and P. Williams. 2012. Bumble bees of the western United States. Washington, DC: USDA Forest Service, Pollinator Partnership. 143 p. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon. Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79.
Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79. Tripoldi, A.D. and A.L. Szalanski. 2015. The bumble bees (Hymenoptera: Apidae: Bombus) of Arkansas, fifty years later. Journal of Melittology 50: doi: http://dx.doi.org/10.17161/jom.v0i50.4834
Tripoldi, A.D. and A.L. Szalanski. 2015. The bumble bees (Hymenoptera: Apidae: Bombus) of Arkansas, fifty years later. Journal of Melittology 50: doi: http://dx.doi.org/10.17161/jom.v0i50.4834 Williams, P., R. Thorp, L. Richardson, and S. Colla. 2014. Bumble Bees of North America. Princeton, NJ: Princeton University Press. 208 p.
Williams, P., R. Thorp, L. Richardson, and S. Colla. 2014. Bumble Bees of North America. Princeton, NJ: Princeton University Press. 208 p.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Benzel, K.R. 2008. Defoliation effects on spotted knapweed seed production and viability. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p.
Benzel, K.R. 2008. Defoliation effects on spotted knapweed seed production and viability. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p. Blicker, P.S. 2000. Nitrate uptake and water use of Centaurea maculosa (Spotted Knapweed) and native grasses. M.Sc. Thesis. Bozeman, MT: Montana State University. 90 p.
Blicker, P.S. 2000. Nitrate uptake and water use of Centaurea maculosa (Spotted Knapweed) and native grasses. M.Sc. Thesis. Bozeman, MT: Montana State University. 90 p. Cheeseman, M.P. 2006. Providing supplement, with or without peg, to reduce the effects of cnicin and enhance grazing of spotted knapweed by sheep and cattle. M.Sc. Thesis. Bozeman, MT: Montana State University. 102 p.
Cheeseman, M.P. 2006. Providing supplement, with or without peg, to reduce the effects of cnicin and enhance grazing of spotted knapweed by sheep and cattle. M.Sc. Thesis. Bozeman, MT: Montana State University. 102 p. Cope, M.G. 1992. Distribution, habitat selection and survival of transplanted Columbian Sharp-tailed Grouse (Tympanuchus phasianellus columbianus) in the Tobacco Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 60 p.
Cope, M.G. 1992. Distribution, habitat selection and survival of transplanted Columbian Sharp-tailed Grouse (Tympanuchus phasianellus columbianus) in the Tobacco Valley, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 60 p. Durham, R. A., D. L. Mummey, L. Shreading, and P.W. Ramsey. 2017. Phenological patterns differ between exotic and native plants: Field observations from the Sapphire Mountains, Montana. Natural Areas Journal, 37(3), 361–381.
Durham, R. A., D. L. Mummey, L. Shreading, and P.W. Ramsey. 2017. Phenological patterns differ between exotic and native plants: Field observations from the Sapphire Mountains, Montana. Natural Areas Journal, 37(3), 361–381. Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p.
Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p. Fraas, W.W. 1992. Bitterbrush growth and reproductive character in relation to browsing in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 137 p.
Fraas, W.W. 1992. Bitterbrush growth and reproductive character in relation to browsing in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 137 p. Fritzen, D.E. 1995. Ecology and behavior of Mule Deer on the Rosebud Coal Mine, Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 143 p.
Fritzen, D.E. 1995. Ecology and behavior of Mule Deer on the Rosebud Coal Mine, Montana. Ph.D. Dissertation. Bozeman, MT: Montana State University. 143 p. Glazier, R.J. 1971. Ecological and morphological relationships of subspecies of Peromyscus maniculatus near St. Mary, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 41 p.
Glazier, R.J. 1971. Ecological and morphological relationships of subspecies of Peromyscus maniculatus near St. Mary, Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 41 p. Gross, J.A. 1998. Elk use of various sized cattle exclosures. M.Sc. Thesis. Bozeman, MT: Montana State University. 40 p.
Gross, J.A. 1998. Elk use of various sized cattle exclosures. M.Sc. Thesis. Bozeman, MT: Montana State University. 40 p. Guenther, G.E. 1989. Ecological relationships of bitterbrush communities on the Mount Haggin Wildlife Management Area. M.Sc. Thesis. Bozeman, MT: Montana State University. 73 p.
Guenther, G.E. 1989. Ecological relationships of bitterbrush communities on the Mount Haggin Wildlife Management Area. M.Sc. Thesis. Bozeman, MT: Montana State University. 73 p. Hansen, Allison, K. 2005. Effects of Spotted Knapweed invasion and restoration treatments on ground beetle diversity abundance and distribution in Rocky Mountain savannas in Montana. M.S. Thesis. University of Montana. Missoula, MT.
Hansen, Allison, K. 2005. Effects of Spotted Knapweed invasion and restoration treatments on ground beetle diversity abundance and distribution in Rocky Mountain savannas in Montana. M.S. Thesis. University of Montana. Missoula, MT. Harvey, S.J. 1990. Responses of steppe plants to gradients of water soil texture and disturbance in Montana, U.S.A. Ph.D. Thesis. Bozeman, MT: Montana State University. 34 p.
Harvey, S.J. 1990. Responses of steppe plants to gradients of water soil texture and disturbance in Montana, U.S.A. Ph.D. Thesis. Bozeman, MT: Montana State University. 34 p. Johnson, T. W. 1982. An analysis of pack and saddle stock grazing areas in the Bob Marshall Wilderness. M.Sc.Thesis. Bozeman, MT: Montana State University. 105 p.
Johnson, T. W. 1982. An analysis of pack and saddle stock grazing areas in the Bob Marshall Wilderness. M.Sc.Thesis. Bozeman, MT: Montana State University. 105 p. Kearing, S.A. 1996. Spotted knapweed (Centaurea maculosa Lam) : water, nutrients, plant competition, bacteria, and the seed head fly (Urophora affinis Frnfd.). M.Sc. Thesis. Bozeman, MT: Montana State University. 66 p.
Kearing, S.A. 1996. Spotted knapweed (Centaurea maculosa Lam) : water, nutrients, plant competition, bacteria, and the seed head fly (Urophora affinis Frnfd.). M.Sc. Thesis. Bozeman, MT: Montana State University. 66 p. King, L.A. 1980. Effects of topsoiling and other reclamation practices on nonseeded species establishment on surface mined land at Colstrip, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 129 p.
King, L.A. 1980. Effects of topsoiling and other reclamation practices on nonseeded species establishment on surface mined land at Colstrip, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 129 p. Lavelle, Darlene. 1986. Use and preference of Spotted Knapweed (Centaurea maculosa) by Elk (Cervus elaphus) and Mule Deer (Odocoileus hemionus) on two winter ranges in western Montana. M.S. Thesis. University of Montana. Missoula, MT.
Lavelle, Darlene. 1986. Use and preference of Spotted Knapweed (Centaurea maculosa) by Elk (Cervus elaphus) and Mule Deer (Odocoileus hemionus) on two winter ranges in western Montana. M.S. Thesis. University of Montana. Missoula, MT. Law, D.J. 1999. A comparison of water table dynamics and soil texture under black cottonwood recent alluvial bar, beaked sedge, and Geyer's/Drummond's willow communities. M.Sc. Thesis. Bozeman, MT: Montana State University. 68 p.
Law, D.J. 1999. A comparison of water table dynamics and soil texture under black cottonwood recent alluvial bar, beaked sedge, and Geyer's/Drummond's willow communities. M.Sc. Thesis. Bozeman, MT: Montana State University. 68 p. Lehnhoff, E.A., L.J.Rew, J.M. Mangold, T. Seipel, and D. Ragen.2019. Integrated management of Cheatgrass (Bromus tectorum) with sheep grazing and herbicide. Agronomy 9, 315
Lehnhoff, E.A., L.J.Rew, J.M. Mangold, T. Seipel, and D. Ragen.2019. Integrated management of Cheatgrass (Bromus tectorum) with sheep grazing and herbicide. Agronomy 9, 315 Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p.
Martinka, R.R. 1970. Structural characteristics and ecological relationships of male blue grouse (Dendragapus obscurus (Say)) territories in southwestern Montana. Ph.D Dissertation. Bozeman, MT: Montana State University. 73 p. Matlock-Cooley, S.J. 1993. Interaction between Deermice, Antelope Bitterbrush, and cattle in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University 84 p.
Matlock-Cooley, S.J. 1993. Interaction between Deermice, Antelope Bitterbrush, and cattle in southwest Montana. M.Sc. Thesis. Bozeman, MT: Montana State University 84 p. Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p.
Meier, G.A. 1997. The colonization of Montana roadsides by native and exotic plants. M.Sc. Thesis. Bozeman, MT: Montana State University. 45 p. Menalled, U.D., S.C. Davis, and J.M. Mangold. 2018. Effects of herbicide management practices used by invasive plant managers on Berteroa incana (Hoary Alyssum) seed biology and control. Invasive Plant and Science Management 11(2):101-106.
Menalled, U.D., S.C. Davis, and J.M. Mangold. 2018. Effects of herbicide management practices used by invasive plant managers on Berteroa incana (Hoary Alyssum) seed biology and control. Invasive Plant and Science Management 11(2):101-106. Mosley, J.C., R.A. Frost, B.L. Roeder, T.K. Mosley, and G. Marks. 2016. Combined herbivory by targeted sheep grazing and biological control insects to suppress spotted knapweed (Centaurea stoebe). Invasive Plant Science and Management 9(1):22-32.
Mosley, J.C., R.A. Frost, B.L. Roeder, T.K. Mosley, and G. Marks. 2016. Combined herbivory by targeted sheep grazing and biological control insects to suppress spotted knapweed (Centaurea stoebe). Invasive Plant Science and Management 9(1):22-32. Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park.
Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park. Park, S.H. 1992. Mode of action of maculosin, a host-specific phytotoxin, produced by Alternaria alternata on spotted knapweed (Centaurea maculosa L.). Ph.D. Dissertation. Bozeman, MT: Montana State University. 94 p.
Park, S.H. 1992. Mode of action of maculosin, a host-specific phytotoxin, produced by Alternaria alternata on spotted knapweed (Centaurea maculosa L.). Ph.D. Dissertation. Bozeman, MT: Montana State University. 94 p. Payson, E. 1996. Gap colonization in grasslands of the Northern Great Plains. M.Sc. Thesis. Bozeman, MT: Montana State University. 37 p.
Payson, E. 1996. Gap colonization in grasslands of the Northern Great Plains. M.Sc. Thesis. Bozeman, MT: Montana State University. 37 p. Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p.
Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p. Rens, E.N. 2003. Geographical analysis of the distribution and spread of invasive plants in the Gardiner Basin, MT. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p.
Rens, E.N. 2003. Geographical analysis of the distribution and spread of invasive plants in the Gardiner Basin, MT. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p. Rinella, M.J., A.D. Knudsen, J.S. Jacobs, and J.M. Mangold. 2020. Seeding causes long-term increases in grass forage production in invaded rangelands. Rangeland and Ecology Management 73(2020): 329-333.
Rinella, M.J., A.D. Knudsen, J.S. Jacobs, and J.M. Mangold. 2020. Seeding causes long-term increases in grass forage production in invaded rangelands. Rangeland and Ecology Management 73(2020): 329-333. Rude, Mark E. 2010. Estimating spotted knapweed intake of sheep using NIRS technology. M.Sc. Thesis. Bozeman, MT: Montana State University. 44 p.
Rude, Mark E. 2010. Estimating spotted knapweed intake of sheep using NIRS technology. M.Sc. Thesis. Bozeman, MT: Montana State University. 44 p. Sater, S. 2022. The insects of Sevenmile Creek, a pictorial guide to their diversity and ecology. Undergraduate Thesis. Helena, MT: Carroll College. 242 p.
Sater, S. 2022. The insects of Sevenmile Creek, a pictorial guide to their diversity and ecology. Undergraduate Thesis. Helena, MT: Carroll College. 242 p. Seipel, T.F. 2006. Plant species diversity in the sagebrush steppe of Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 87 p.
Seipel, T.F. 2006. Plant species diversity in the sagebrush steppe of Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 87 p. Story, J.M. 1976. A study of Urophora affinis (Diptera: Tephritidae) released on spotted knapweed in Western Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 77 p.
Story, J.M. 1976. A study of Urophora affinis (Diptera: Tephritidae) released on spotted knapweed in Western Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 77 p. Thrift, Brian D. 2005. Summer diets of sheep grazing spotted knapweed-infested foothill rangeland in Western Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 59 p.
Thrift, Brian D. 2005. Summer diets of sheep grazing spotted knapweed-infested foothill rangeland in Western Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 59 p. Tierney, K.R. 2013. Effects of training on cattle grazing spotted knapweed and Canada thistle. M.Sc. Thesis. Bozeman, MT: Montana State University. 75 p.
Tierney, K.R. 2013. Effects of training on cattle grazing spotted knapweed and Canada thistle. M.Sc. Thesis. Bozeman, MT: Montana State University. 75 p. Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp.
Tuinstra, K. E. 1967. Vegetation of the floodplains and first terraces of Rock Creek near Red Lodge, Montana. Ph.D dissertation. Montana State University, Bozeman 110 pp. Weigand, J.P. 1994. Range use and interspecific competition of Rocky Mountain bighorn sheep in the Highland Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 75 p.
Weigand, J.P. 1994. Range use and interspecific competition of Rocky Mountain bighorn sheep in the Highland Mountains, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 75 p. Willard, E.E., D.J. Bedunah, and C.L. Marcum. 1988. Impacts and potential Impacts of spotted knapweed (Centaurea maculosa) on forest and range lands in western Montana. Unpubl. Rep., Montana For. and Conserve Exp. Sta., University of Montana, Missoula.
Willard, E.E., D.J. Bedunah, and C.L. Marcum. 1988. Impacts and potential Impacts of spotted knapweed (Centaurea maculosa) on forest and range lands in western Montana. Unpubl. Rep., Montana For. and Conserve Exp. Sta., University of Montana, Missoula. Zapatka, T.P. 1963. Some results of two limited hunting seasons on hen Pheasants in north central Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 26 p.
Zapatka, T.P. 1963. Some results of two limited hunting seasons on hen Pheasants in north central Montana. M.Sc. Thesis. Bozeman, Montana: Montana State University. 26 p.
- Web Search Engines for Articles on "Spotted Knapweed"





