View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Hairy Water Fern - Marsilea vestita
Native Species
Global Rank:
G5
State Rank:
S3
C-value:
4
Agency Status
USFWS:
USFS:
BLM:
External Links
General Description
PLANTS: Aquatic or amphibious perennials that grow in colonies from shallow, branched rhizomes. Sources: Johnson in Flora of North America (FNA) 1993; Lesica et al. 2022
LEAVES: Alternate and clover-like. Leaf petioles are longer than leaf blades and are stiffly erect in water, or procumbent on land. Leaf petiole is longer than leaf blades. Blades are palmately divided into 4-equal, broadly ovate, leaflets (pinnae). Each pinnae is sparsely hairy (pubescent) to glabrous. Sources: Johnson in Flora of North America (FNA) 1993; Lesica et al. 2022
SPOROCARPS: Brown, often hairy, globose to ovate structures borne on short stalks at the base of a leaf petiole. They are 3-6 mm long, nut-like in character, and slightly nod. The sporocarp has 2 prominent teeth. The lower tooth is about 0.5 mm high. The upper tooth is longer and pointed (acute). Sources: Johnson in FNA 1993; Lesica et al. 2022
The specific epithet oligospora is a combination of the Greek oligos, meaning “oligarchy” or “few”, and the Latin spora, meaning “spores,” “seeds,” or “to sow”. Marsilea is for Count Luigi Marsigli, an Italian naturalist and mycologist of the 18th century (Giblin et al. [eds.] 2018).
Phenology
Sporocarps are produced from April through October (Johnson in Flora of North America [FNA] 2019).
Diagnostic Characteristics
Montana has two native
Marsilea species. The plants resemble a clover because of their leaves - 4 broadly ovate, whorled leaflets (pinnae) - but they produce spores (not seeds) and are classified as fern-like. Yet, they are not ferns, in part, because their spore-producing structures (sporangium) are found inside a sporocarp and not on the underside of a leaf.
Hairy Water Fern-
Marsilea vestita, native
*Leaf petioles: 1-20 cm long, but could be up to 40cm when floating.
*Pinnae: 3-19 mm long and sparsely pubescent to glabrous.
*Sporocarps: 3-6 mm long and slightly nod at maturity. Hairy (pubescent) but becoming glabrous with age.
*Sporocarp teeth: The lower tooth is usually about 0.5 mm long while the upper tooth is longer and acute.
Pepperwort-
Marsilea oligospora, native, SOC
*Leaf petioles: 3-6 cm long
*Pinnae: 6-15 mm long and pilose.
*Sporocarps: 5-6 mm long and nod at maturity. Hairy (pubescent) but becoming glabrous with age.
*Sporocarp teeth: The lower tooth is usually about 0.5 mm long, and curves away from the sporocarp. The upper is blunt and smaller or absent.
Range Comments
Hairy Water Fern ranges from British Columbia to Saskatchewan south to Mexico and South America (Lesica et al. 2012).
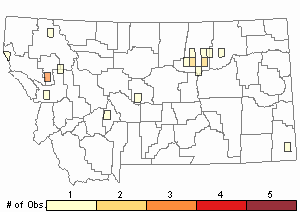
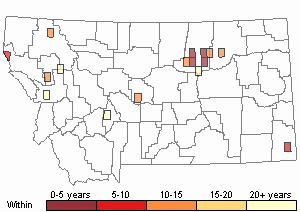
Observations in Montana Natural Heritage Program Database
Number of Observations: 20
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
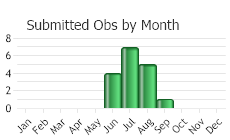
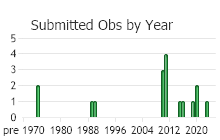
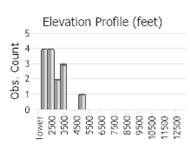 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
The preferred habitat of Hairy Water Fern is fresh to saline, shallow water of ponds, reservoirs, and sloughs found in plains and valleys of Montana (Lesica et al. 2012). Hairy Water Fern requires habitats that have both wet and dry seasonal periods (Chau and Reyes 2014).
Ecology
LIGHT AND DEVELOPMENT
When grown in the absence of light, the photosynthetic pinnae of Hairy water fern develop but do not expand and instead become elongated, linear, and pale (Gaudet 1965). However, the development of stomata and the elongation of the petioles suggest that leaf development originates from within the leaf itself and is not controlled by the rest of the plant. Individuals grown without light also exhibit lower growth rates and lower foliar weight.
NYCTINASTY
Marsilea species are the only group of ferns that exhibit nyctinasty, or daily movement of leaf orientation (Petlewski et al. 2019). During the day, they assume a more or less horizontal position in order to maximize capture of sunlight and then change orientation by moving into a vertical position at night (Minorsky 2019). The mechanism by which this occurs is the pulvinus joint found at the swollen base of the leaf stalk which moves with water inflow or outflow to the pulvinus motor cells (Iwai et al. 2016). Nyctinastic movement in Marsilea species matches the rhythm of transpiration through opening and closing of stomata (Iwai et al. 2016).
PARASITISM
Parasitism by numerous insect species, including weevils from the genus Echinocnemus and several wasp species, has been observed on Marsilea mollis in Arizona (Mauz et al. 2009). Endalus celatus, another weevil species, has been associated with M. mucronata (Mauz et al. 2009). In these cases, adults feed on the edges of the leaves and females deposit their eggs in the sporocarps (Board et al. 1971). After hatching, larvae feed on the spores inside the sporocarps and then pupate in the resulting empty cavity (Board et al. 1971). Similar weevil and wasp interactions may exist for other Marsilea species.
Reproductive Characteristics
Marsilea species are fern-like vascular plants that reproduce from spores; thus, the terminology below is often specific to ferns.
SPOROCARPS
Sporocarps are the fruit case which contain sporangia and the spores; they are unique to members of the Family Marsilieace. Visually, sporocarps are nut-like or bean-shaped structures that attach by a short stalk to the base of a leaf petiole (Nagalingum et al. 2006). When young, sporocarps are softer and may be green but become very hard and brown with age. Within the thick, hard wall of the mature sporocarp, are specialized reproductive structures that are enfolded and fused to contain two rows of sori (Pryer and Hearn 2009). The sori attach to a mucilaginous ring (Bilderback 1978). Because of these unique structures, leaves are not needed to house sporangium, as is the situation for true-ferns. The formation of sporocarps also allow the plants to become sexually mature earlier than other true-fern species (Pryer and Hearn 2009). This is especially advantageous for an organism that occupies habitats with uncertain conditions of moisture. Once the moisture is favorable for reproduction, then the sporocarp of an individual plant is ready to germinate (Pryer and Hearn 2009).
SORUS
The sorus is a cluster of sporangia, plural, with each sporangium, singular, containing the spores. Each sorus is surrounded by a thin shield (indusium) and contains two types of sporangium (bisporaginate): megasporangium produce haploid spores that develop into female gametophytes, and microsporangium produce haploid spores that develop into male gametophytes (Nagalingum et al. 2007). The indusia of adjacent sori are partially fused.
LIFE CYCLE
The life cycle of Marsilea is very unique in that they rely on the occurrence of both wet and dry periods to complete their reproductive life cycle (Chau and Reyes 2014). Germination occurs during the rainy season. Precipitation allows the sporocarp's contents (mature sori and mucilaginous ring) to become hydrated. The mucilaginous ring that surrounds the sori expands dramatically and ruptures the sporocarp, breaking it into valves, and carrying the sori to water (Johnson in FNA 1993; Nagalingum et al. 2007). From the sori, spores germinate (gametophyte growth) and fertilization occurs immediately (Johnson in FNA 1993). The gametophytic phase takes up to two days after which the sporophyte quickly develops rhizomes and leaves (Pryer and Hearn 2009). Development of new sporocarps follows the end of the growing season when the habitat dries completely (Pryer and Hearn 2009). In California, Marsilea oligospora reproduces while the vernal pond is inundated. Further, this species demonstrated an extreme specialization in being only found in vernal ponds that were inundated for a period of at least 212 days (Gosejohan et al. 2017). Sporocarps of Marsilea oligospora are remarkably tolerant of desiccation because successful germination was demonstrated even after 100 years (Chau 2013).
Management
Conservation measures to retain Marsilea vestita populations require maintaining ephemeral habitats. Extensive observation data of Marsilea vestita is needed to determine how management affects its status and biology in Montana.
Some insights for management may come from a closely related species that is endemic to Hawaii, Marsilea villosa (Wester et al. 2006). Marsilea villosa, has experienced a drastic decline in its range despite attempted conservation and management of populations (Wester et al. 2006). This is most likely due to its narrow habitat requirements combined with human alteration and aggressive invasive species. Off-road vehicles, trampling and grazing by cattle, and invasive grasses have been suggested as contributors to the plant's dwindling distribution. At many sites aggressive, invasive grass species have out-competed Marsilea villosa. Managers have found that Marsilea villosa plants responded positively to mowing because it reduced competition caused by the taller growth of grasses (Wester et al. 2006).
Stewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Bilderback, D.E. 1978. The DEcelopment of the Sporocarp of Marsilea vestita. American Journal of Botany, 65(6): 629-637.
Bilderback, D.E. 1978. The DEcelopment of the Sporocarp of Marsilea vestita. American Journal of Botany, 65(6): 629-637. Chau, Marian M., and Whitney R. Reyes. 2014. Effects of Light, Flooding, and Weeding on Experimental Restoration of an Endangered Hawaiian Fern. January. Restoration Ecology. Volume 22, Number 1, pp. 107-116.
Chau, Marian M., and Whitney R. Reyes. 2014. Effects of Light, Flooding, and Weeding on Experimental Restoration of an Endangered Hawaiian Fern. January. Restoration Ecology. Volume 22, Number 1, pp. 107-116. Chau, Marian M., Whitney R. Reyes, and Tom A. Ranker. 2013. Ecological Factors Influencing Growth of the Endangered Hawaiian Fern Marsilea villosa (Marsileaceae) and Implications for Conservation Management. American Journal of Botany, 100(8), pp. 1532-1543.
Chau, Marian M., Whitney R. Reyes, and Tom A. Ranker. 2013. Ecological Factors Influencing Growth of the Endangered Hawaiian Fern Marsilea villosa (Marsileaceae) and Implications for Conservation Management. American Journal of Botany, 100(8), pp. 1532-1543. Flora of North America Editorial Committee. 1993. Flora of North America north of Mexico. Vol. 2. Pteridophytes and gymnosperms. Oxford Univ. Press, New York. xvi + 475 pp.
Flora of North America Editorial Committee. 1993. Flora of North America north of Mexico. Vol. 2. Pteridophytes and gymnosperms. Oxford Univ. Press, New York. xvi + 475 pp. Gosejohan, Meredith C., Peter J. Weisberg, and Kyle E. Merriam. 2016. Hydrologic Influences on Plant Community Structure in Vernal Pools of Northeastern California. Wetlands, Society of Wetland Scientists. DOI 10.1007/s13157-016-0863-3
Gosejohan, Meredith C., Peter J. Weisberg, and Kyle E. Merriam. 2016. Hydrologic Influences on Plant Community Structure in Vernal Pools of Northeastern California. Wetlands, Society of Wetland Scientists. DOI 10.1007/s13157-016-0863-3 Johnson, David M. 1985. Marsilea quadrifolia and M. vestita in the Floras of Kansas and Missouri. American Fern Journal 75, pp. 28-29.
Johnson, David M. 1985. Marsilea quadrifolia and M. vestita in the Floras of Kansas and Missouri. American Fern Journal 75, pp. 28-29. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Mauz, Kathryn, and John Reeder. 2009. Marsilea Mollis (Marsileaceae) Sporocarps and Associated Insect Parasitism in Southern Arizona. September. Western North American Naturalist, Volume 69, Number 3, pp. 382-387.
Mauz, Kathryn, and John Reeder. 2009. Marsilea Mollis (Marsileaceae) Sporocarps and Associated Insect Parasitism in Southern Arizona. September. Western North American Naturalist, Volume 69, Number 3, pp. 382-387. Pryer, Kathleen M., and David J. Hearn. 2009. Evolution of Leaf Form in Marsileaceous Ferns: Evidence for Heterochrony. Evolution, February, Volume 63, Number 2, pp. 498-513.
Pryer, Kathleen M., and David J. Hearn. 2009. Evolution of Leaf Form in Marsileaceous Ferns: Evidence for Heterochrony. Evolution, February, Volume 63, Number 2, pp. 498-513.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p.
Eggers, M.J.S. 2005. Riparian vegetation of the Montana Yellowstone and cattle grazing impacts thereon. M.Sc. Thesis. Montana State University, Bozeman, MT. 125 p. Merriman, J.W., C.W. Boal, T.L. Bashore, P.J. Zwank, and D.B. Wester. 2006. Abundance of diurnal raptors in relation to prairie dog colonies: implications for bird-aircraft strike hazard. Journal of Wildlife Management. 71(3).
Merriman, J.W., C.W. Boal, T.L. Bashore, P.J. Zwank, and D.B. Wester. 2006. Abundance of diurnal raptors in relation to prairie dog colonies: implications for bird-aircraft strike hazard. Journal of Wildlife Management. 71(3).
- Web Search Engines for Articles on "Hairy Water Fern"





