View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Longleaf Oregon-grape - Berberis nervosa
Other Names:
Mahonia nervosa
State Rank Reason (see State Rank above)
Berberis nervosa is disjunct in northern Idaho. In Montana it is known from 2-3 locations in Sanders County, of which one population in 2001 is reported to have over 1,000 plants. Additional data on locations and population sizes are greatly needed.
- Details on Status Ranking and Review
Population Size
ScoreD - 1,000 - 2,500 individuals
Range Extent
ScoreA - <100 sq km (< ~40 sq mi)
Area of Occupancy
ScoreA - 1 4-km2 grid cell
Number of Populations
ScoreA - 1 - 5
Number of Occurrences or Percent Area with Good Viability / Ecological Integrity
ScoreB - Very few (1-3) occurrences with excellent or good viability or ecological integrity
Environmental Specificity
ScoreC - Moderate. Generalist or community with some key requirements scarce
Threats
ScoreD - Low
CommentNo known threats.
General Description
Plants: Longleaf Oregon-grape (
Berberis nervosa) is a low shrub with yellow inner bark and 10-30 cm tall aboveground stems (Hitchcock and Cronquist 1973). It can form extensive colonies of clones connected by rhizomes (underground stems) (Huffman and Tappeiner 1997). Its leaves and bud scales are leathery, and the bud scales are 2-4 cm long and remain on the plants (Hitchcock and Cronquist 1973).
Leaves: Pinnate, on wiry petioles that widen at the base (Drum 2000). Evergreen and alternate, 20-40 cm long, and divided into 9-19 oblong or ovate leaflets (Lesica et al. 2012; Pojar and MacKinnon 1994). The leaflets have spiny margins and are glossy above and below, without hairs (Lesica et al. 2012).
Inflorescence: Terminal racemes, up to 20 cm long and composed of showy yellow flowers (Hitchcock and Cronquist 1973; Lesica et al. 2012).
(Contribution of Lesica et al. adapted from
Lesica et al. 2012. Manual of Montana Vascular Plants. BRIT Press. Fort Worth, TX)
Diagnostic Characteristics
Our other Oregon-grapes (
Berberis repens and
Berberis aquifolium) are also spiny-leaved, evergreen shrubs, but they average fewer leaflets (up to 9 but less than 11), have smaller bud scales (less than 1 cm long), and are pinnately-nerved instead of palmately-nerved (Lesica et al. 2012; Hitchcock and Cronquist 1973).
Berberis repens is much more widespread in Montana (Kartesz 2015).
(Contribution of Lesica et al. adapted from
Lesica et al. 2012. Manual of Montana Vascular Plants. BRIT Press. Fort Worth, TX)
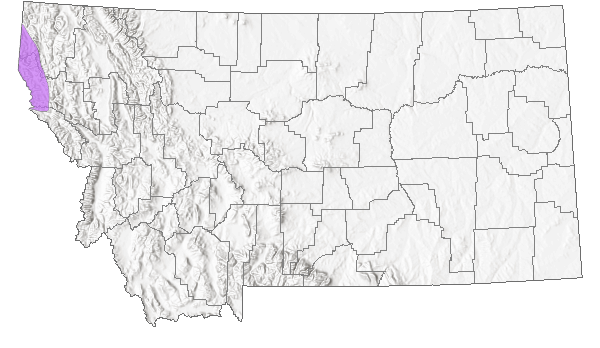
Species Range
Montana Range
Range Descriptions
 Native
Native
Range Comments
BC to CA, disjunct in ID and Sanders County, MT (Lesica et al. 2012. Manual of Montana Vascular Plants. BRIT Press. Fort Worth, TX).
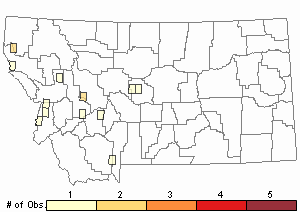
Observations in Montana Natural Heritage Program Database
Number of Observations: 5
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
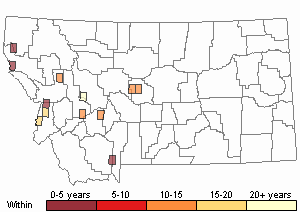
Recency
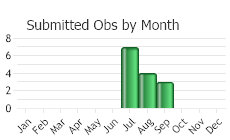
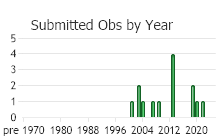
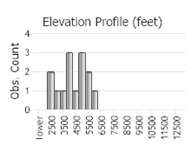 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Wet coniferous forest in the valleys (Lesica et al. 2012). Prefers moister sites than
Berberis aquifolium (Petrini et al. 1982).
(Contribution of Lesica et al. adapted from
Lesica et al. 2012. Manual of Montana Vascular Plants. BRIT Press. Fort Worth, TX)
National Vegetation Classification System Groups Associated with this Species
Forest and Woodland
Montane - Subalpine Forest and Woodland
Ecology
An introduced slug,
Arion rufus, is known to feed on
Berberis nervosa leaves, preferring younger growth but also feeding on the undersides of older leaves (Lauren and Whitlow 2012). In a nine-month experiment in western Washington, higher slug densities correlated with statistically significant decreases in relative leaf area (Lauren and Whitlow 2012). The researchers also noted relatively more non-slug invertebrates in plots with more slugs, though at levels that were not statistically significant. They hypothesized that slug excrement was responsible for this effect. Potentially, increases in invertebrates could lead to higher soil nitrate levels and increased growth, but this study did not detect that. The researchers pointed out that those effects might only show up in a longer duration study (Lauren and Whitlow 2012).
Newly emerged leaves are eaten by deer, elk, and goats (Drum 2000). Individual leaves can persist for up to five years (Huffman and Tappeiner 1997). While the vegetation of many broad-leaved evergreens responds to wounding by folding in at the wound margin and then gradually developing wound cork, mature
Berberis nervosa leaves do not. Instead, they die back irregularly to the nearest major vein, and often exhibit fungal growth along the dying margin (Wylie 1931). Young
B. nervosa leaves, on the other hand, behave similarly to those of other broad-leaved evergreens: the epidermis folds inwards, providing temporary protection from infection while a layer of wound cork grows (Wylie 1931).
In southwest British Columbia, which receives an average of 150 centimeters of precipitation per year, a greenhouse study of forest seed banks found that only one
B. nervosa germinated out of 16,289 seeds (all plant species) (McGee and Feller 1993). In this study,
B. nervosa was a dominant understory plant in drier or moderately moist immature, successional forests and was not dominant in moist forests or in powerline rights-of-way that were cleared 2-3.5 years prior.
A study in Oregon’s Coast Range, which receives an average of 178 centimeters of precipitation per year, found that
B. nervosa seedlings were substantially denser, with significantly more biomass, in Douglas-fir stands that had been thinned 5-8 years before, compared to similar-aged (35 to 55 year old) stands that had not been thinned (Huffman and Tappeiner 1997). There were over twice the number of current-year rhizome extensions, and growth of aerial stems was significantly greater. However, there was not a significant difference in the length of current-year rhizome extensions or in the rhizome length between ramets (genetically identical individuals in the same population) in thinned versus unthinned stands.
Another Oregon study found endophytic fungi in 99% of
B. nervosa leaves sampled from five sites across a range of humidity and canopy density conditions (Petrini et al. 1982). Fungal endophytes live inside plant tissue without causing disease symptoms, and some are known to deter herbivores or prevent colonization by pathogens (Petrini et al. 1982; Carroll 1988). Six different taxa of endophytes were noted in
B. nervosa, and 56% of leaves contained at least two different species. One endophyte,
Leptothyrium berberidis, was found in a particularly high number of
B. nervosa leaves, and less commonly in the leaves of two other studied plants, suggesting that it had some degree of host specificity (Petrini et al. 1982). This contrasts with broad generalist endophytes, such as
Phomopsis sp. – found within many plants – which occurred in a much smaller percentage of
B. nervosa leaves (Carroll 1988; Petrini et al. 1982).
POLLINATORS The following animal species have been reported as pollinators of this plant species or its genus where their geographic ranges overlap:
Bombus vagans,
Bombus melanopygus,
Bombus mixtus,
Bombus terricola,
Bombus bimaculatus,
Bombus griseocollis, and
Bombus impatiens (Plath 1934, Macior 1968, Thorp et al. 1983, Colla and Dumesh 2010).
Reproductive Characteristics
Flowers: Three yellow, deciduous bract-like structures surround 6 yellow sepals and 6 smaller yellow petals. The 6 stamens are opposite the petals, and the ovary is superior (Lesica et al. 2012; Hitchcock and Cronquist 1973).
Fruit: Berries are 8-11 mm long and blue with a whitish bloom. They have few seeds and can be eaten but are quite tart (Lesica et al. 2012; Pojar and MacKinnon 1994). Seeds germinate in the spring when soil is cold (Richo Cech
in Drum 2000).
(Contribution of Lesica et al. adapted from
Lesica et al. 2012. Manual of Montana Vascular Plants. BRIT Press. Fort Worth, TX)
Management
Longleaf Oregon-grape is a coastal disjunct in northern Idaho and northwestern Montana. In Idaho, some botanists interpret its presence to be a signal that other coastal disjunct species could be present.
Coastal native peoples used the bright yellow underground parts of Berberis spp. as a dye for baskets (Pojar and MacKinnon 1994). Herbalists report success using the rhizomes and stems of Berberis spp. in decoctions and tinctures to treat various conditions, including bacterial infections and Giardia parasitism (Drum 2000). Since Berberis nervosa has limited distribution in Montana, and is at the edge of its range (Kartesz 2015), Berberis repens would probably be a better species to use here. In Pacific Northwest forests where this species is abundant, it can regenerate well from a careful harvest of stems and rhizomes for herbal use; re-growth is much slower in drier areas with marginal habitat (Drum 2000).
B. nervosa primarily reproduces vegetatively, via rhizomes (McKenzie and Halpern 1999). It can be propagated by burying rhizome pieces horizontally or by vertically planting a 10-15 cm long stem that has buds below the ground (Drum 2000). Seeds that are separated from the fruit and washed can be planted outside in the fall to early spring timeframe. Seeds germinate in early spring when the soil is cold; germination can take 70 days (Richo Cech in Drum 2000).
Stewardship Responsibility
Threats or Limiting Factors
STATE THREAT SCORE REASON
Threat impact not assigned because threats are not known (MTNHP Threat Assessment 2021).
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Carroll, G. 1988. Fungal Endophytes in Stems and Leaves: From Latent Pathogen to Mutualistic Symbiont. Ecology 69(1):2-9.
Carroll, G. 1988. Fungal Endophytes in Stems and Leaves: From Latent Pathogen to Mutualistic Symbiont. Ecology 69(1):2-9. Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:39-68. Drum, R. W. 2000. Oregon Grape - Mahonia spp. IN Gladstar, R. and P. Hirsch (eds.) Planting the Future: Saving Our Medicinal Herbs. Rochester, VT: Healing Arts Press. 310 p.
Drum, R. W. 2000. Oregon Grape - Mahonia spp. IN Gladstar, R. and P. Hirsch (eds.) Planting the Future: Saving Our Medicinal Herbs. Rochester, VT: Healing Arts Press. 310 p. Hitchcock, L. C. and A. Cronquist. 1973. Flora of the Pacific Northwest. University of Washington Press, Seattle. 730 pp.
Hitchcock, L. C. and A. Cronquist. 1973. Flora of the Pacific Northwest. University of Washington Press, Seattle. 730 pp. Huffman, D.W.; Tappeiner, J.C., II. 1997. Clonal expansion and seedling recruitment of Oregon grape (Berberis nervosa) in Douglas-fir (Pseudotsuga menziesii) forests: comparisons with salal (Gaultheria shallon). Canadian Journal of Forest Research. 27: 1788-1793.
Huffman, D.W.; Tappeiner, J.C., II. 1997. Clonal expansion and seedling recruitment of Oregon grape (Berberis nervosa) in Douglas-fir (Pseudotsuga menziesii) forests: comparisons with salal (Gaultheria shallon). Canadian Journal of Forest Research. 27: 1788-1793. Kartesz, J.T. 2015. The Biota of North America Program (BONAP) North American Plant Atlas. Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2015. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)].
Kartesz, J.T. 2015. The Biota of North America Program (BONAP) North American Plant Atlas. Chapel Hill, N.C. [maps generated from Kartesz, J.T. 2015. Floristic Synthesis of North America, Version 1.0. Biota of North America Program (BONAP). (in press)]. Lauren, H. Z. G. and W. L. Whitlow. 2012. Ecological Effects of Invasive Slugs, Arion rufus, on Native Cascade Oregon Grape, Mahonia nervosa. Northwest Science 86(1): 1-8.
Lauren, H. Z. G. and W. L. Whitlow. 2012. Ecological Effects of Invasive Slugs, Arion rufus, on Native Cascade Oregon Grape, Mahonia nervosa. Northwest Science 86(1): 1-8. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Macior, L.M. 1968. Bombus (Hymenoptera, Apidae) queen foraging in relation to vernal pollination in Wisconsin. Ecology 49:20-25.
Macior, L.M. 1968. Bombus (Hymenoptera, Apidae) queen foraging in relation to vernal pollination in Wisconsin. Ecology 49:20-25. McGee, A. and M. C. Feller. 1993. Seed banks of forested and disturbed soils in southwestern British Columbia. Can.J. Bot. 71:1574-1583.
McGee, A. and M. C. Feller. 1993. Seed banks of forested and disturbed soils in southwestern British Columbia. Can.J. Bot. 71:1574-1583. McKenzie, D. and C.B. Halpern. 1999. Modeling the distribution of shrub species in Pacific northwest forests. Forest Ecology and Management 114:293-307
McKenzie, D. and C.B. Halpern. 1999. Modeling the distribution of shrub species in Pacific northwest forests. Forest Ecology and Management 114:293-307 MTNHP Threat Assessment. 2021. State Threat Score Assignment and Assessment of Reported Threats from 2006 to 2021 for State-listed Vascular Plants. Botany Program, Montana Natural Heritage Program, Helena, Montana.
MTNHP Threat Assessment. 2021. State Threat Score Assignment and Assessment of Reported Threats from 2006 to 2021 for State-listed Vascular Plants. Botany Program, Montana Natural Heritage Program, Helena, Montana. Petrini, O., J. Stone, and F.E. Carroll. 1982. Endophytic fungi in evergreen shrubs in western Oregon: A preliminary study. Can. J. Bot. 60: 789-796.
Petrini, O., J. Stone, and F.E. Carroll. 1982. Endophytic fungi in evergreen shrubs in western Oregon: A preliminary study. Can. J. Bot. 60: 789-796. Plath, O.E. 1934. Bumblebees and their ways. New York, NY: Macmillan Company. 201 p.
Plath, O.E. 1934. Bumblebees and their ways. New York, NY: Macmillan Company. 201 p. Pojar, J. and A. MacKinnon, eds. 1994. Plants of the Pacific Northwest coast: Washington, Oregon, British Columbia & Alaska. Lone Pine Publishing, Vancouver, British Columbia and Renton, Washington. 527 pp.
Pojar, J. and A. MacKinnon, eds. 1994. Plants of the Pacific Northwest coast: Washington, Oregon, British Columbia & Alaska. Lone Pine Publishing, Vancouver, British Columbia and Renton, Washington. 527 pp. Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79.
Thorp, R.W., D.S. Horning, and L.L. Dunning. 1983. Bumble bees and cuckoo bumble bees of California (Hymenoptera: Apidae). Bulletin of the California Insect Survey 23:1-79. Wylie, R. B. 1931. Cicatrization of Foliage Leaves II. Wound Responses of Certain Broad-Leaved Evergreens. Botanical Gazette 92(3):279-295.
Wylie, R. B. 1931. Cicatrization of Foliage Leaves II. Wound Responses of Certain Broad-Leaved Evergreens. Botanical Gazette 92(3):279-295.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
- Web Search Engines for Articles on "Longleaf Oregon-grape"





