View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Prairie Tree Cricket - Oecanthus argentinus
General Description
Preface to the Genus Oecanthus The following is taken from Fulton (1915, June 1926), Walker (1962), and Funk (1982). Species in this genus are divided into 4 natural groups based on their morphology, color and habits. These groups are
niveus,
releyi,
nigricornus, and
varicornis. Only two groups occur in Montana, with 4 species in the
nigricornus group and one species,
Snowy Tree Cricket (
O. fultoni), in the
releyi group.
Generally, tree crickets are characterized by their long, slender shape, pale colors and arboreal habits. The tegmina (forewings) of males are broad and flat, and female wings are long and narrow and wrapped closely to the body. Their legs are very slender, armed with two rows of minute teeth interspersed with a number of delicate spines. The tarsi (feet) are three-segmented with the second segment being small and compressed. The antennae are longer than the body, filiform and often ornamented with distinctive black markings. The first two basal antennal segments are distinctively marked with spots, which are a convenient diagnostic character for species identification (see illustrations for each species).
The Prairie Tree Cricket’s wings tend to be longer and narrower than other species. The head has a yellowish cast and distal areas of the legs and antennae are dark (Fulton 1915 and June 1926, Hebard 1928, Walker 1963, Helfer 1971, Capinera and Sechrist 1982, Vickery and Kevan 1985, Capinera et al. 2004, and Scott 2010).
Song production in OecanthusSong is produced only by males, which raise their forewings (tegmina) perpendicular to the body and vibrate them in a transverse direction, so that the overlapping inner portions rub against each other. The sound-producing mechanism is located near the base of the wings. It is a broad expanded distal area which serves as a resonator to increase sound volume. A short prominent transverse vein occurs beneath forming a rasp-like structure about 1-1.5 mm wide with 20-50+ short teeth, depending upon the species, inclined toward the opposite wing. The number of teeth is a diagnostic feature. The right wing, termed the file, bears a series of downward projecting teeth, overlaps the left, termed the scraper. When the tegmina are raised and alternately opened and closed, the song is produced only on the closing stroke. Due to this partial wing-motion cycle, cricket songs are constructed of sound pulses. If a cricket opens and closes its wings many times in succession, it produces a trill. If the wings are opened and closed a few times, pauses, and repeats the song again, it produces a chirp. Songs vary according to ambient temperatures and is distinctive by sound analysis between species.
Due to the Prairie Tree Cricket’s narrow front wings, its song is considered fainter in volume compared to other Montana species. The song quality has been described as prolonged, unvaried, monotonous and somewhat tinny. In late fall, some individuals develop a raspy undertone (Fulton June 1926, and Walker 1963).
Phenology
Overwinters in the egg stage. Eggs hatch in late June and adults occur from late July to first frost in fall. Only one generation is produced per year in Montana, two generations at more southerly latitudes (Vickery and Kevan 1985).
Diagnostic Characteristics
The following comes from Fulton (1915, June 1926), Hebard (1928), Walker (1963), Helfer (1971), Capinera and Sechrist (1982), Vickery and Kevan (1985), Bland (2003), Capinera et al. (2004), and Scott (2010). The body length of the Prairie Tree Cricket for males and females is 13–15 mm. The black marks on the ventral surface of the 1st antenna segment are broad with an attached backward-pointing horizontal mark. In some rare individuals, this mark may be separated by a space nearly as wide as the longitudinal mark. The two marks on the 2nd segment are united or narrowly separated. Microscopically, the stridulatory file on the male tegmina measures about 1 mm and possesses 42-53 teeth with an average of ±46.
All
Oecanthus species are similar in general form, appearance and behavior as noted above. The
Forbe's Tree Cricket (
O. forbesi) and
Black-horned Tree Cricket (
O. nigrocornis) have similar looking, thick markings on the 1st and 2nd antennal segments. Separation of the two species from the Prairie Tree Cricket is by song, as
Forbe's and
Black-horned Tree Cricket have a faster song. Many tree crickets could also be confused for katydids which are placed in a different family, the
Tettigoniidae (Collins 2021 in Walker
Singing Insects of North America, and Scott 2010).
Species Range
Montana Range
Range Descriptions
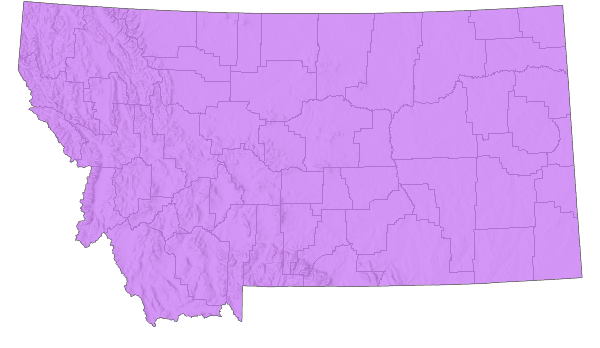
 Native
Native
Range Comments
The following is taken from Transeau (1935), Walker (1963), Gleason and Cronquist (1964), Stuckey (1981), Vickery and Kevan (1985), Scott (2010), and Meszaros and Denny (2017). Widespread throughout the western U.S. from the southern western provinces of Canada to Mexico, and from the West Coast to an extension across the mid-West into the western half of Ohio. This extension of the western grassland across Iowa, Illinois, Indiana into western Ohio, is correlated with the post-glacial retreat of the Wisconsin glacier during the Pleistocene. Around 4,000 years ago, warming took place, known as the “Xerothermic Period,” and prairie plants of the Great Plains extended their ranges eastward forming what is termed the Prairie Peninsula (Transeau 1935, and Gleason and Cronquist 1964). The western Prairie Tree Cricket expanded its range to follow this habitat. Since 1954, there is evidence that this species has expanded further eastward in response to prairie-like habitats created by agriculture and urban/suburban infrastructures. The Prairie Tree Cricket has been collected in 4 counties, but is probably more widespread than presently reported.
Observations in Montana Natural Heritage Program Database
Number of Observations: 3
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Inhabits fencerows and other areas of low, brushy, herbaceous and woody vegetation consisting of such plants as ragweed (Ambrosia), goldenrod (Solidago), aster, gumweed (Grindelia sp.), fleabane (Erigeron), blackberry, and rose etc. (Fulton June 1926, and Vickery and Kevan 1985).
Food Habits
The food habits of all the Oecanthus species consists of a great variety of both plant and animal origin, but the food items must be soft enough to be masticated by their weak jaws. One of their dietary mainstays is aphids. They also consume plant material by chewing holes in leaves, outer tissue of stems, the anthers of some plant flowers, chew holes in ripe fruit (apples, peaches, plums, etc.) to feed on the inner pulp, and eat fruiting bodies of fungi. The Prairie Tree Cricket favors gumweed, Grindelia sp. (Fulton June 1926, Vickery and Kevan 1985, and Himmelman 2009).
Reproductive Characteristics
Reproduction in Oecanthus
The following is taken from Fulton (1915, June 1926), Walker (1962), and Funk (1982). The mating and reproduction habits of all Oecanthus species are generally similar in behavior and complexity. When a female is attracted to a singing male, she approaches him from the rear because that is direction most of the sound travels and amplified by his wings. When the male senses the female’s approach, he stops singing, turns around and touches her with his antennae. It is thought that this “tasting” of her confirms she is the same species and an appropriate mate. The next step in the courtship ritual, (can vary slightly among species) the male again sings and rocks from side to side or pounds his abdomen on the substrate. If the female is receptive to his “romantic overtures,” she climbs onto his back, the male raises his tegmina at a 45° angle, and she begins feeding on a substance secreted from a glandular cavity, called the metanotal, metathoracic or Hancock’s gland (which he called the “alluring gland”) located between the wing bases. The secretions empty into a pit at the base of the male’s hind wings where they are accessible to the female once she is in the copulatory position. After she begins to feed, she allows the male to mate with her. During copulation, the male reaches his abdomen back, the female bends her abdomen downward connecting their genitalia, and the male passes a sperm-containing spermatophore to the female. The spermatophore is ovoid and possesses a thin, threadlike tube which enters the female, leaving the spermatophore to dangle outside her genital opening and, when internally attached, sperm begins to move through the tube. After copulation, the female always eats the spermatophore because it is thought to contain nutrients which may enhance egg production. The female remains astride the male for 5-20 minutes or more, continuing to feed from the metanotal gland. When she stops feeding, she dismounts, but the male may attempt to resume active courtship by repeatedly backing under the female until she either remounts or moves away. If remounting occurs, the renewed courtship may require another 20 minutes or more. Such resumed courtship and mating is thought to ensure that large numbers of sperm pass from his spermatophore into the female’s sperm-storage organ called the spermatheca. When the female is ready to lay eggs, sperm are released from the spermatheca and fertilize the eggs as they pass down the oviduct. The female selects a suitable egg-laying site on a woody or herbaceous plant to oviposit and chews a small hole in the stem, then moves forward slightly, arches her back, bringing her ovipositor perpendicular to the hole moving it up and down drilling the hole with thrusts and turning it around by twisting her abdomen. The drilling time may require 5-25 minutes, depending upon the plant’s wood or fiber density. Once the hole is drilled to the proper size and depth, the female moves an egg down her ovipositor, fills the hole with a mucilaginous substance, removes the ovipositor, and neatly caps the hole with chewed plant material. The entire process from chewing the bark to sealing the hole may take a quarter hour to nearly a full hour.
The Prairie Tree Cricket lays its eggs in the stems of most plants found in its habitat and diet, again seeming to favor gumweed. However, the stems of chosen oviposition sites must have a pith with stalks durable enough to stand throughout the winter. Woody plants seem less desirable as oviposition sites. This species selects stems less than 5 mm in diameter, thus laying mostly in the terminal growths and scattered vertically. Upon hatching, the Prairie Tree Cricket passes through 5 instars before reaching the adult stage (Fulton June 1926, and Vickery and Kevan 1985).
Stewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Bland, R.G. 2003. The Orthoptera of Michigan—Biology, Keys, and Descriptions of Grasshoppers, Katydids, and Crickets. East Lansing, MI: Michigan State University Extension, Bulletin E-2815. 221 p.
Bland, R.G. 2003. The Orthoptera of Michigan—Biology, Keys, and Descriptions of Grasshoppers, Katydids, and Crickets. East Lansing, MI: Michigan State University Extension, Bulletin E-2815. 221 p. Capinera, J.L. and T.S. Sechrist. 1982. Grasshoppers of Colorado: Identification, Biology, and Management. Fort Collins, CO: Colorado State University Experiment Station, Bulletin 584S. 161 p.
Capinera, J.L. and T.S. Sechrist. 1982. Grasshoppers of Colorado: Identification, Biology, and Management. Fort Collins, CO: Colorado State University Experiment Station, Bulletin 584S. 161 p. Capinera, J.L., R.D. Scott, and T.J. Walker. 2004. Field Guide to Grasshoppers, Katydids, and Crickets of the United States. Ithaca, NY. Cornell University Press.
Capinera, J.L., R.D. Scott, and T.J. Walker. 2004. Field Guide to Grasshoppers, Katydids, and Crickets of the United States. Ithaca, NY. Cornell University Press. Collins, N. 2021. Tree cricket, tree cricket photos, videos, data. Accessed 17 March 2021. http://www.oecanthinae.com/
Collins, N. 2021. Tree cricket, tree cricket photos, videos, data. Accessed 17 March 2021. http://www.oecanthinae.com/ Fulton, B.B. 1915. The tree crickets of New York: life history and bionomics. New York Agricultural Experiment Station Technical Bulletin 42.
Fulton, B.B. 1915. The tree crickets of New York: life history and bionomics. New York Agricultural Experiment Station Technical Bulletin 42. Fulton, B.B. June 1926. The tree crickets of Oregon. Oregon Agricultural College Experiment Station, Station Bulletin 223. 20 p.
Fulton, B.B. June 1926. The tree crickets of Oregon. Oregon Agricultural College Experiment Station, Station Bulletin 223. 20 p. Fulton, B.B. October 1926. Geographical variation in the Nigrocornis group of Oecanthus (Orthoptera). Iowa State College Journal of Science 1(1):42-46.
Fulton, B.B. October 1926. Geographical variation in the Nigrocornis group of Oecanthus (Orthoptera). Iowa State College Journal of Science 1(1):42-46. Funk, D.H. 1982. The mating of tree crickets. Scientific American 261(2):50-59.
Funk, D.H. 1982. The mating of tree crickets. Scientific American 261(2):50-59. Gleason, H.A. and A. Cronquist. 1964. The natural geography of plants. New York, NY: Columbia University Press. 420 p.
Gleason, H.A. and A. Cronquist. 1964. The natural geography of plants. New York, NY: Columbia University Press. 420 p. Hebard, M. 1928. The Orthoptera of Montana. Proceedings of the Academy of Natural Sciences of Philadelphia, Vol. 80:211-306.
Hebard, M. 1928. The Orthoptera of Montana. Proceedings of the Academy of Natural Sciences of Philadelphia, Vol. 80:211-306. Helfer, J.R. 1971. How to Know the Grasshoppers, Crickets, Cockroaches, and Their Allies. Revised edition (out of print), Mineola, NY: Dover Publications.
Helfer, J.R. 1971. How to Know the Grasshoppers, Crickets, Cockroaches, and Their Allies. Revised edition (out of print), Mineola, NY: Dover Publications. Himmelman, J. 2009. Guide to Night-Singing Insects of the Northeast. Mechanicsburg, PA: Stackpole Books. 160 p.
Himmelman, J. 2009. Guide to Night-Singing Insects of the Northeast. Mechanicsburg, PA: Stackpole Books. 160 p. Meszaros, G. and G.L. Denny. 2017. The prairie peninsula. Kent, OH: The Kent State University Press. 136 p.
Meszaros, G. and G.L. Denny. 2017. The prairie peninsula. Kent, OH: The Kent State University Press. 136 p. Scott, R.D. 2010. Montana Grasshoppers, Katydids, and Crickets A Pictorial Field Guide to the Orthoptera. MagpieMTGraphics, Billings, MT.
Scott, R.D. 2010. Montana Grasshoppers, Katydids, and Crickets A Pictorial Field Guide to the Orthoptera. MagpieMTGraphics, Billings, MT. Stuckey, R.L. 1981. Origin and development of the concept of the prairie peninsula. Ohio Biological Survey Biological Notes (15):4-23.
Stuckey, R.L. 1981. Origin and development of the concept of the prairie peninsula. Ohio Biological Survey Biological Notes (15):4-23. Transeau, E.N. 1935. The prairie peninsula. Ecology 16(3):423-437.
Transeau, E.N. 1935. The prairie peninsula. Ecology 16(3):423-437. Vickery, V. R. and D. K. M. Kevan. 1985. The grasshopper, crickets, and related insects of Canada and adjacent regions. Biosystematics Research Institute, Ottawa, Ontario. Publication Number 1777. 918 pp.
Vickery, V. R. and D. K. M. Kevan. 1985. The grasshopper, crickets, and related insects of Canada and adjacent regions. Biosystematics Research Institute, Ottawa, Ontario. Publication Number 1777. 918 pp. Walker T.J.(ed.). 2020. Singing insects of North America. Accessed 10 February 2021. https://orthsoc.org/sina/
Walker T.J.(ed.). 2020. Singing insects of North America. Accessed 10 February 2021. https://orthsoc.org/sina/ Walker, T.J. 1962. Factors responsible for intraspecific variation in the calling songs of crickets. Evolution 16:407-428.
Walker, T.J. 1962. Factors responsible for intraspecific variation in the calling songs of crickets. Evolution 16:407-428.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Alexander, R.D. and D. Otte. 1967. The evolution of genitalia and mating behavior in crickets (Gryllidae) and other Orthoptera. Ann Arbor, MI: University of Michigan. Misc. publications, Museum of Zoology, No. 133. 69 p.
Alexander, R.D. and D. Otte. 1967. The evolution of genitalia and mating behavior in crickets (Gryllidae) and other Orthoptera. Ann Arbor, MI: University of Michigan. Misc. publications, Museum of Zoology, No. 133. 69 p. Brown, W.D. 1997. Courtship feeding in tree crickets increases insemination and reproductive life span. Animal Behavior 54:1369-1382.
Brown, W.D. 1997. Courtship feeding in tree crickets increases insemination and reproductive life span. Animal Behavior 54:1369-1382. Dethier, V.G. 1992. Crickets and Katydids, Concerts and Solos. Cambridge, MA: Harvard University Press. 140 p.
Dethier, V.G. 1992. Crickets and Katydids, Concerts and Solos. Cambridge, MA: Harvard University Press. 140 p. Elliott, L. and W. Hershberger. 2007. The songs of insects. New York, NY: Houghton Mifflin Harcourt. 227 p.
Elliott, L. and W. Hershberger. 2007. The songs of insects. New York, NY: Houghton Mifflin Harcourt. 227 p. Gangwere, S.K., M.C. Muralirangan, and M. Muralirangan (eds). 1997. The bionomics of grasshoppers, katydids and their kin. New York, NY: CAB International. 528 p.
Gangwere, S.K., M.C. Muralirangan, and M. Muralirangan (eds). 1997. The bionomics of grasshoppers, katydids and their kin. New York, NY: CAB International. 528 p. Himmelman, J. 2011. Cricket radio: tuning in the night-singing insects. Cambridge, MA: The Belknap Press of Harvard University Press. 272 p.
Himmelman, J. 2011. Cricket radio: tuning in the night-singing insects. Cambridge, MA: The Belknap Press of Harvard University Press. 272 p. Huber, F. and J. Thorson. 1985. Cricket auditory communication. Scientific American 253(6):60-73.
Huber, F. and J. Thorson. 1985. Cricket auditory communication. Scientific American 253(6):60-73. Walker, T.J. 1963. The taxonomy and calling songs of United States tree crickets (Orthopters:Gryllidae:Oecanthus). The nigricornis group of the genus Oecanthus. Annals of the Entomological Society of America 56(6):772-789.
Walker, T.J. 1963. The taxonomy and calling songs of United States tree crickets (Orthopters:Gryllidae:Oecanthus). The nigricornis group of the genus Oecanthus. Annals of the Entomological Society of America 56(6):772-789. Walker, T.J. 1967. The metanotal gland as a taxonomic character in Oecanthus of the United States. Proceedings of the Entomological Society of Washington 69(2):151-167.
Walker, T.J. 1967. The metanotal gland as a taxonomic character in Oecanthus of the United States. Proceedings of the Entomological Society of Washington 69(2):151-167. Walker, T.J. 1969. Acoustic synchrony: two mechanisms in the snowy tree cricket. Science 166:891-894.
Walker, T.J. 1969. Acoustic synchrony: two mechanisms in the snowy tree cricket. Science 166:891-894.
- Web Search Engines for Articles on "Prairie Tree Cricket"
- Additional Sources of Information Related to "Insects"





