View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Dalmatian Toadflax Stem-boring Weevil - Mecinus janthiniformis
General Description
Following many North American introductions of what was thought to be a single species, Mecinus janthinus, for use as a biocontrol agent against exotic toadflax (Linaria spp.), Mecinus janthiniformis is now recognized as a distinct cryptic species of M. janthinus (Tosevski et al. 2011). Because of the early failure to recognize the two species, some of what follows in the species account may pertain to both species.
[From Carney et al. 2004; Schat et al. 2007; Tosevski et al. 2011] Length 3.1-6.0 mm (rostrum excluded); mean length of males 4.1 mm (range 3.2-6.0 mm), females 4.1 mm (range 3.3-6.0 mm). Integument black except pronotum and elytra dark blue with metallic reflections. Rostrum in lateral view moderately and regularly curved, moderately long in male (about 0.80-0.88X as long as pronotum length), somewhat longer and apical portion more strongly curved in female (about 1.03-1.09X as long as pronotum length); rostrum moderately striate-punctured with numerous stout setae to apex (male) or striate-puntured with stout setae only in basal half then almost smooth (female). Pronotum sculpture formed by deep and small punctures more densely apressed, intervals between punctures narrow, smooth and shiny, clearly visible between sparse seta-like long white scales, widest at basal third. Elytra very long, about 2X as long as wide, with interstriae roughly sculptured and covered with recumbent seta-like white scales almost completely arranged in two rows (not a single median row). Profemora with distinct sharp tooth in male, unarmed in female. Aedeagus with sides slightly more abruptly narrowed in subapical part (not gradually narrowing in distal third), toward apex ending in form of subtruncate tip.
Phenology
In Eurasia, adults overwinter in host plant stems, emerge early April, egg-laying early May to mid-July (Tosevski et al. 2011). In North America, adults active early April to mid-August (overwinter other months), eggs early May to early July, larvae mid-May to mid-August, pupae mid-June to mid-September (Jeanneret and Schroeder 1992; Wilson et al. 2005; McClay and Hughes 2007).
Diagnostic Characteristics
Probably best differentiated from closely-related species by host plant specificity, larger body size (length), apical portion of female rostrum in lateral view more curved, punctures on pronotum slightly smaller and more densely adpressed, scales of elytral interstriae denser and arranged in two rows on parts of several interstriae (not one median row), aedeagus with sides more abruptly narrowed (not parallel) and ending in form of subtruncate tip (not subacute tip).
Species Range
Montana Range
Range Descriptions

 Non-native
Non-native
Range Comments
Native Range: Eastern portion of central and southeastern Europe (Hungary, Serbia, Romania, Bulgaria, Mecedonia, Greece), probably widely distributed throughout native range of host plant Linaria genistifolia (Ukraine, Moldova Republic, western borders of south Russia to south-central Siberia, northern Caucasus) and Linaria dalmatica (southeast Europe); to about 1500 m elevation (Jeanerret and Schroeder 1992; Tosevski et al. 2011).
North America: Introduced in 1991-1999 (Wilson et al. 2005; Tosevski et al. 2011). In Canada, established on Linaria dalmatica in British Columbia, and Alberta (Vujnovic and Wein 1997; Wilson et al. 2005), which is the primary host plant (Tosevski et al. 2011). This species established on Linaria dalmatica south in western US to California, Colorado, and Utah (Wilson et al. 2005; Breiter and Seastedt 2007; Jamieson et al. 2012; Hinz et al. 2014). Major releases also reported for Idaho, Montana, North Dakota, Oregon, South Dakota, Washington, and Wyoming (Wilson et al. 2005), but these likely included Mecinus janthinus; first release in Montana was 1996, with additional releases across the state during 1997-2000 (Sing et al. 2008).
Observations in Montana Natural Heritage Program Database
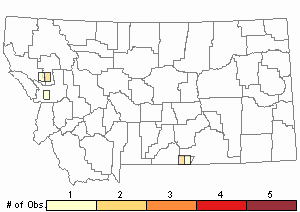
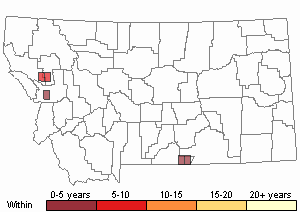
Number of Observations: 10
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
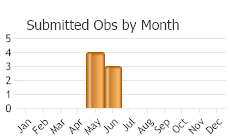
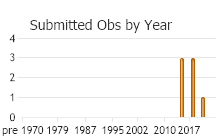
 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Migration
Non-migratory; release populations can disperse > 3.2 km in 4 years (Wilson et al. 2005), > 10.0 km in 10 years (Van Hezewijk et al. 2010).
Habitat
Arid forest, montane meadows and pastures, rangeland supporting large-stemmed toadflaxes (Linaria) (Wilson et al. 2005; Tosevski et al. 2011).
Food Habits
Host plants for larvae and adults
Linaria (toadflax), primarily
Dalmatian Toadflax (Linaria dalmatica and L. genistifolia) (Tosevski et al. 2011). Earlier reports (prior to split of
Mencinus janthinus into two cryptic species) included additional toadflax species as host plants in Eurasia (e.g.,
L. repens,
L. vulgaris and
Chaenorrhinum minus). Feeding choice trials showed no significant difference in number of eggs laid and number of feeding marks on
L. vulgaris from Canada,
L. vulgaris from the native range in Europe, and
L. dalmatica from Canada, implying both species of
Linaria established in North America were suitable host plants for
M. janthiniformis. Adults also infrequently nibble on other plant species (
Collinsia,
Cymbalaria,
Digitalis,
Gratiola,
Kickxia,
Mimulus,
Scrophularia,
Solidago,
Veronica) (Jeanerret and Schroeder 1992). Also able to feed and complete development in captivity on native
Nuttallanthus canadensis and
Sairopcarpus virga, possibly also
Nuttallanthus texanus in the field, as well as exotic
Antirrhinum and some additional
Linaria species (Wilson et al. 2005; Breiter and Seastedt 2007; McClay and Hughes 2007; Hinz et al. 2014). Larvae feed in host plant stems, adults feed on leaves and in stems (Jeanerret and Schroeder 1992; Wilson et al. 2005; Schat et al. 2011).
Reproductive Characteristics
Copulation occurs shortly after adults emerge in spring from overwintering in dry host plant stems. Females lay eggs in shallow holes chewed by female on actively growing host plant shoots typically on the upper part of central stem or basal part of lateral flowering branches, 1 egg per hole, 1 egg (rarely 2 eggs) per shoot, however multiple females may lay eggs on same stem. Holes with eggs have lids to protect egg from desiccation and predation. Females lay about 1 egg/day under laboratory conditions, 45 to > 100 eggs total. Eggs hatch in 6-7 days under laboratory conditions, develop from L1 instar to L3 instar and pupae in 23-40 days, larvae develop in a slightly elongated gall-like alteration (a response to oviposition and larval development), feed and mine central part of stem and gall-like tissue usually for 1-2 cm, pupate in cell within host plant stem, adults emerge from pupae in another 27-29 days, but remain in pupal cells and hibernate. Only one generation produced/year (Jeanneret and Schroeder 1992; Saner et al. 1994; Wilson et al. 2005; Tosevski et al. 2011). Overwinter mortality of adults varies, depending on exposure to low temperatures and reduced snow cover; number of dead adults can exceed live adults per stem (De Clerck-Floate and Miller 2002; McClay and Hughes 2007; Sing et al. 2008).
Management
Biological control agents are most effective when integrated with other biocontrol and traditional methods. With
Dalmatian Toadflax (Linaria dalmatica), eight introduced insect species attack various parts or stages of the host plant (including flowers, seed capsules, foliage, and roots).
Mecinus janthiniformis, a stem-boring agent, does not appear to shift feeding preference to most native North American (non-target)
Linaria and related Scrophulariaceae species when given opportunity to do so (Sing et al. 2005). However, given the potential to develop on non-target native host plants, some of which are rare species in some states, additional testing under multiple-choice conditions and postrelease monitoring is required to determine host ranges of this biocontrol beetle (Hinz et al. 2014).
The following general suggestions (from Wilson et al. 2005) may help insure successful collection and establishment of biocontrol insects for toadflax:
1) Determine beforehand the efficacy (ability to control the target weed), host plant specificity, and biocontrol availability for the biocontrol insects to be used.
2) Select release sites based on their desired function. Field nursery sites (for future biocontrol collection and redistribution) should exceed 10 acres with fairly continuous distribution of toadflax, be safe from disturbance, and be accessible for regular monitoring. Sites for long-term monitoring should be buffered from other weed management programs and disturbance (grazing) for up to 10 years. Sites not intended for monitoring or biocontrol collection should be sufficiently large and free from disturbance to provide the agents the best possible chance to survive and flourish.
3) Agents should be stored and transported in sturdy containers that are kept shaded, cool, and well ventilated. An example is a pint-sized, nonwaxed, paperboard carton. Plastic containers can work with large holes cut in lid and protected with mesh screening. Avoid glass or metal containers. Prepare containers for agents by adding cut leafy stem pieces of toadflax for food, shelter, moisture. Do not add water. Transport containers in a large cooler with pre-frozen icepacks.
Specifically to
Mecinus janthiniformis, adult beetles are the life stage to transfer and introduce. Adults can be obtained at sites with established beetle populations by tapping plants over tubs (tray sampling) to dislodge beetles, or sweep netting, then aspirated and transferred to containers. At least 200 adult beetles are recommended for initial release of stem-boring weevils, which can be collected in 30 minutes or less if the source population is appropriately large to support redistribution collections. Collect adults that are actively feeding and mating on top portions of plants. Transport and release agents as soon as possible (preferably within 24 hours). Release should occur in good weather in cooler early morning or evening hours. Release on the ground at the base of stems in a dense stand of young host plants in full sunlight.
Melissa Maggio-Kassner is the coordinator for the Montana Biological Weed Control Project. She can be reached at (406) 258-4223 or
mmaggio@missoulaeduplace.orgUseful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesUseful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteAquatic Invasive SpeciesStewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Breiter, N.C. and T.R. Seastedt. 2007. Postrelease evaluation of Mecinus janthinus host specificity, a biological control agent for invasive toadflax (Linaria spp.)
Breiter, N.C. and T.R. Seastedt. 2007. Postrelease evaluation of Mecinus janthinus host specificity, a biological control agent for invasive toadflax (Linaria spp.) Carney, V.A, J. Rau, S.M. Little, and R.A. DeClerck-Floate. 2004. Rapid differentiation of the sexes of adult Mecinus janthinus (Coleoptera: Curculionidae) based on external leg morphology. Canadian Entomologist. 136:835-837.
Carney, V.A, J. Rau, S.M. Little, and R.A. DeClerck-Floate. 2004. Rapid differentiation of the sexes of adult Mecinus janthinus (Coleoptera: Curculionidae) based on external leg morphology. Canadian Entomologist. 136:835-837. DeClerck-Floate, R. and V. Miller. 2002. Overwintering mortality of and host attack by the stem-boring weevil, Mecinus janthinus Germar, on Dalmatian toadflax (Linaria dalmatica (L.) Mill.) in western Canada. Biological Control. 24:65-74.
DeClerck-Floate, R. and V. Miller. 2002. Overwintering mortality of and host attack by the stem-boring weevil, Mecinus janthinus Germar, on Dalmatian toadflax (Linaria dalmatica (L.) Mill.) in western Canada. Biological Control. 24:65-74. Hinz, H.L., M. Schwarzlander, A. Gassmann,and R.S. Bourchie. 2014. Successes we may not have had: a retrospective analysis of selected weed biological control agents in the United States. Invasive Plant Science and Management. 7:565-579.
Hinz, H.L., M. Schwarzlander, A. Gassmann,and R.S. Bourchie. 2014. Successes we may not have had: a retrospective analysis of selected weed biological control agents in the United States. Invasive Plant Science and Management. 7:565-579. Jamieson, M.A., D. Knochel, A.Q. Manrique, and T.R. Seastedt. 2012. Top-down and bottom-up controls on Dalmatian toadflax (Linaria dalmatica) performance along the Colorado Front Range, USA. Plant Science. 213:185-195.
Jamieson, M.A., D. Knochel, A.Q. Manrique, and T.R. Seastedt. 2012. Top-down and bottom-up controls on Dalmatian toadflax (Linaria dalmatica) performance along the Colorado Front Range, USA. Plant Science. 213:185-195. Jeanneret, P. and D. Schroeder. 1992. Biology and host specificity of Mecinus janthinus Germar (Col.: Curculionidae), a candidate for the biological control of yellow and Dalmatian toadflax, Linaria vulgaris (L.) Mill. and Linaria dalmatica (L.) Mill. (Scrophulariaceae) in North America. Biocontrol Science and Technology. 2:25-34.
Jeanneret, P. and D. Schroeder. 1992. Biology and host specificity of Mecinus janthinus Germar (Col.: Curculionidae), a candidate for the biological control of yellow and Dalmatian toadflax, Linaria vulgaris (L.) Mill. and Linaria dalmatica (L.) Mill. (Scrophulariaceae) in North America. Biocontrol Science and Technology. 2:25-34. McClay, A.S. and R.B. Hughes. 2007. Temperature and host-plant effects on development and population growth of Mecinus janthinus (Coleoptera: Curculionidae), a biological control agent for invasive Linaria spp. Biological Control 40:405-410.
McClay, A.S. and R.B. Hughes. 2007. Temperature and host-plant effects on development and population growth of Mecinus janthinus (Coleoptera: Curculionidae), a biological control agent for invasive Linaria spp. Biological Control 40:405-410. Saner, M.A., P. Jeanneret, and H. Muller-Scharer. 1994. Interaction among two biological control agents and the developmental stage of their target weed, Dalmatian toadflax, Linaria (L.) Mill. (Scrophulariaceae). Biocontrol Science and Technology. 4:215-222.
Saner, M.A., P. Jeanneret, and H. Muller-Scharer. 1994. Interaction among two biological control agents and the developmental stage of their target weed, Dalmatian toadflax, Linaria (L.) Mill. (Scrophulariaceae). Biocontrol Science and Technology. 4:215-222. Schat, M., S.E. Sing, and R.K.D. Peterson. 2007. External rostral characters for differentiation of sexes in the biological control agent Mecinus janthinus (Coleoptera: Curculionidae). Canadian Entomologist. 139:354-357.
Schat, M., S.E. Sing, and R.K.D. Peterson. 2007. External rostral characters for differentiation of sexes in the biological control agent Mecinus janthinus (Coleoptera: Curculionidae). Canadian Entomologist. 139:354-357. Schat, M., S.E. Sing, R.K.D. Peterson, F.D. Menalled,and D.K. Weaver. 2011. Growth inhibition of Dalmatian toadflax, Linaria dalmatica (L.) Miller, in response to herbivory by the biological control agent Mecinus janthinus Germar. Journal of Entomological Science. 46:232.246.
Schat, M., S.E. Sing, R.K.D. Peterson, F.D. Menalled,and D.K. Weaver. 2011. Growth inhibition of Dalmatian toadflax, Linaria dalmatica (L.) Miller, in response to herbivory by the biological control agent Mecinus janthinus Germar. Journal of Entomological Science. 46:232.246. Sing, S.E., D.K. Weaver, R.M. Nowierski, and G.P. Markin. 2008. Long-term field evaluation of Mecinus janthinus releases against Dalmatian toadflax in Montana (USA). Proceedings of the XII International Symposium on Biological Control of Weeds.12:620-624.
Sing, S.E., D.K. Weaver, R.M. Nowierski, and G.P. Markin. 2008. Long-term field evaluation of Mecinus janthinus releases against Dalmatian toadflax in Montana (USA). Proceedings of the XII International Symposium on Biological Control of Weeds.12:620-624. Sing, S.E., R.K.D. Peterson, D.K. Weaver, R.W. Hansen, and G.P. Markin 2005. A retrospective analysis of known and potential risks associated with exotic toadflax-feeding insects
Sing, S.E., R.K.D. Peterson, D.K. Weaver, R.W. Hansen, and G.P. Markin 2005. A retrospective analysis of known and potential risks associated with exotic toadflax-feeding insects Toševski, I., R. Caldara, J. Jović, G. Hernández-Vera, C. Baviera, A. Gassmann, and B.C. Emerson. 2011. Morphological, molecular and biological evidence reveal two cryptic species in Mecinus janthinus Germar (Coleoptera, Curculionidae), a successful biological control agent of Dalmatian toadflax, Linaria dalmatica (Lamiales, Plantaginaceae). Systematic Entomology (2011), DOI: 10.1111/j.1365-3113.2011.00593.
Toševski, I., R. Caldara, J. Jović, G. Hernández-Vera, C. Baviera, A. Gassmann, and B.C. Emerson. 2011. Morphological, molecular and biological evidence reveal two cryptic species in Mecinus janthinus Germar (Coleoptera, Curculionidae), a successful biological control agent of Dalmatian toadflax, Linaria dalmatica (Lamiales, Plantaginaceae). Systematic Entomology (2011), DOI: 10.1111/j.1365-3113.2011.00593. Van Hezewijk, B.H., R.S. Bourchier, and R.A. De Clerck-Floate. 2010. Regional-scale impact of the weed biocontrol agent Mecinus janthinus on Dalmatian toadflax (Linaria dalmatica). Biological Control. 55:197-202.
Van Hezewijk, B.H., R.S. Bourchier, and R.A. De Clerck-Floate. 2010. Regional-scale impact of the weed biocontrol agent Mecinus janthinus on Dalmatian toadflax (Linaria dalmatica). Biological Control. 55:197-202. Vujnovic, K. and R.W. Wein. 1997. The biology of Canadian weeds. 106. Linaria dalmatica (L.) Mill. Canadian Journal of Plant Science. 77:483-491.
Vujnovic, K. and R.W. Wein. 1997. The biology of Canadian weeds. 106. Linaria dalmatica (L.) Mill. Canadian Journal of Plant Science. 77:483-491. Wilson, L.M., S.E. Sing, G.L. Piper, R.W. Hansen, R. De Clerck-Floate, D.K. MacKinnon, and C.B. Randall. 2005. Biology and biological control of Dalamatian and Yellow Toadflax. Morgantown, WV: USDA Forest SErvice. FHTET-05-13. 116p.
Wilson, L.M., S.E. Sing, G.L. Piper, R.W. Hansen, R. De Clerck-Floate, D.K. MacKinnon, and C.B. Randall. 2005. Biology and biological control of Dalamatian and Yellow Toadflax. Morgantown, WV: USDA Forest SErvice. FHTET-05-13. 116p.
- Web Search Engines for Articles on "Dalmatian Toadflax Stem-boring Weevil"
- Additional Sources of Information Related to "Insects"





