View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Black Dot Leafy Spurge Flea Beetle - Aphthona nigriscutis
General Description
Genus Aphthona: Front carinate, frontal tubercles well developed and clearly marginated, antennae longer than half the body length, prothorax broader than long, pronotum lacking both longitudinal and transverse grooves, elytra wider at base than pronotum, elytral punctation confused or (sometimes) in irregular rows, procoxal cavities open, tibial spurs simple and inserted at outer corner of tibia, first hind tarsi usually distinctly shorter than 1/2 length of hind tibia. Male with first segment of front tarsus enlarged (not enlarged in female), posterior margin of last visible abdominal sternite distinctly lobate (evenly rounded in female), males smaller and more slender on average than females; female antennae proportionally shorter (LeSage and Paquin 1996).
Aphthona nigriscutis: adults 3.0-3.5 mm in body length; dorsal and ventral body surfaces brown or brownish; scutellum dark brown, contrasting with paler color of pronotum and elytra; hind femora yellowish; tip of male aedeagus nipple-shaped; female spermathecae declivous portion of spermathecal duct bent at obtuse angle from longitudinal axis of recepticle viewpoint, dorsal surface of recepticle concave; female styli with pair of short setae at apex, dark brown markings usually sharply contrasting over pale areas of styli (LeSage and Paquin 1996; Rees et al. 1996).
Phenology
Adults June to August, eggs June to August, larvae July to May of following year, pupae early spring to May (Rees et al. 1996; Jackson 1997; Lajeunesse et al. 1997; Skinner et al. 2006).
Diagnostic Characteristics
See General Description (above) for distinguishing Aphthona from other beetles. A. nigriscutis differs from A. lacertosa externally by being brown with a contrasting black dot behind the thorax at the leading edge of the wings instead of having an overall black appearance (except the legs) with metallic blue to dark green reflections of the body.
Species Range
Montana Range
Range Descriptions
 Non-native
Non-native
Range Comments
Native Distribiton: Central and eastern Europe, Russian, central Asia, eastern Siberia (Butler et al. 2006).
North America: Released in Canada in 1983, the US in 1989 (Jackson 1997). In the US through 1996, established in Colorado, Idaho, Iowa, Michigan, Minnesota, Montana, Nebraska, Nevada, New Mexico, North Dakota, Oregon, South Dakota, and Washington (Rees et al. 1996; Hansen et al. 1997). In Montana, established in at least 21 counties mostly in the western 2/3 of the state, as far east as Big Horn, Musselshell, Petroleum and Yellowstone counties, but also Carter County (Hansen et al. 1997; Butler et al. 2006; Wacker and Butler 2006).
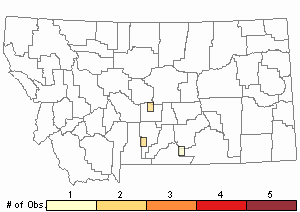
Observations in Montana Natural Heritage Program Database
Number of Observations: 6
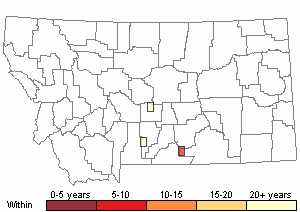
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
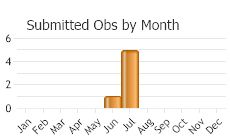
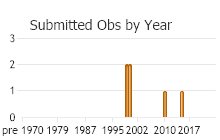
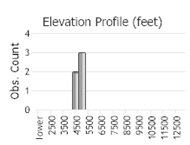 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Migration
Not described; apparently non-migratory. Dispersal rare in Alberta between release plots and non-release plots 100-200 m apart and imbedded in shrubland, more likely to disperse in grasslands, with greater dispersal capabilities by females (Jonsen et al. 2001); dispersal also rare between release plots and non-release plots 100-1000 m apart in Montana and South Dakota (Butler et al. 2006).
Habitat
Uncultivated fields, pastures, rangelands, and disturbed sites (such as railroad right-of-ways) where
Leafy Spurge (Euphorbia esula) and related
Euphorbia species present, particularly in dry open habitats on hilltops with well-drained and coarse sandy soils (Gassmann et al. 1996; Rees et al. 1996; Lajeunesse et al. 1997; Lym and Nelson 2000); beetle densities highest in shrub and grassland habitats, lowest in woodland and river bottom habitats in western North Dakota (Larson and Grace 2004). For xeric sites in eastern Montana with annual precipitation of 280-380 mm, beetle densities greatest in sites with high annual precipitation; beetle density not related to sand content of soils nor total stem density of leafy spurge, but was positively related to density of flowering leafy spurge stems and distance from beetle release points (Jacobs et al. 2001).
Food Habits
Host plants restricted to
Euphorbia. Reported larval host plants in Europe include several species in the subgenus
Esula, especially
E. cyparissias,
E. esula,
E. lathyris,
E. myrsinites, and
E. seguieriana, to a lesser extent
E. amygdaloides,
E. oblongata,
E. polychroma, and
E. virgata; also some feeding on the non-euphorbs
Linum usitatissmum and
Vinca rosea (Gassmann et al. 1996). Larvae feed in root hairs and roots (Rees et al. 1996; Lajeunesse et al. 1997; Skinner et al. 2004). In transfer tests, L1 instar larvae placed on 8 other
Euphorbia species and 7 genera (8 species) of other potential host plants developed to L3 instar only on the
Euphorbia species mentioned above (Gassmann et al. 1996). Adults raised from
E. cyparissias show a preference for
E. cyparissias,
E. esula,
E. myrsinites,
E. seguieriana, and
E. virgata, to a lesser extent
E. amygdaloides,
E. lathyris,
E. oblongata,
E. peplus,
E. polychroma, and
E. virgata, with occasional nibling by adults on six other
Euphorbia species as well as the euphorbs
Aleurites fordii and
Ricinus communis; also some nibbling on the non-euphorbs
Lactuca sativa and
Rheum rhaponticum (Gassmann et al. 1996). Adults feed on host plant leaves and flowers (Rees et al. 1996; Lajeunesse et al. 1997). Does not feed on native (non-target)
Euphorbia brachycera in South Dakota (Wacker and Butler 2006).
Reproductive Characteristics
Median longevity of adult males in captivity 221 days (25-387 days), of adult females 124 days (47-258 days); about 60% of adults live > 120 days (Jackson 1997). Adult females deposit eggs underground in small batches near host plant root and stem over several months during summer (Rees 1996; Lajeunesse et al. 1997). Egg laying begins 4-16 days after eclosion (emergence from pupae), each female produces about 250-800 eggs (range: 103-1157 eggs) over a period of 60-200 days, most egg-laying occurs within the first 100 days, daily production about 2-6 eggs; in captivity about 60% of eggs hatch. Eggs hatch in about 9-11 days after oviposition, most eggs (perhaps > 80%) produce females (Jackson 1997). Upon hatch, L1 instars migrate to root hairs and feed in roots until winter dormancy as L3 instar, in early spring L3 instar resumes feeding then moves from roots to soil and forms a cell in which to pupate (Rees et al. 1996; Lajeunesse et al. 1997; Skinner et al. 2004).
Management
Biological control agents are most effective when integrated with other biocontrol and traditional methods, such as herbicides, grazing, fire, and reseeding (Lajeunesse et al. 1997; Lym 2005). When using fire, beetles are more succesful when released on plots in habitats already suitable for beetles and burned prior to their release, possibly because fire reduces accumulated litter and further bares the soil (Fellows and Newton 1999).
Aphthona nicriscutis does not appear to shift feeding preference to native North American (non-target)
Euphorbia when given opportunity to do so (Wacker and Butler 2006).
The following general suggestions (from Lajeunesse et al. 1997) may help insure successful collection and establishment of biocontrol insects:
1) Determine beforehand the habitat requirements for biocontrol insects to be used. Avoid sites with high ant and grasshopper populations, and seek areas free from grazing, herbicide or pesticide use. Initial release sites should be protected for up to 10 years, secondary sites need less protected time.
2) Collection should be made with minimum stress to the insects. Beetles can be collected by using a sweep net through the upper portions of leafy spurge plants 8-10 times, then dumping content into a container.
3) Release insects as quickly as possible. If moved more than 80 km or held for more than a few hours, the biocontrol species should be sorted out from other species of arthropods captured during sweeping. Biocontrol insects should be kept cool during transport through use of a cooler with refrigerated (not frozen) coolant packs.
4) Release biocontrol insects during the cool parts of a day by sprinkling over a small area (10-15 square meters) on a leafy spurge infestation of moderate density. Avoid tall, dense stands that may provide too much shade and high humidity.
5) Permits are required to transport biocontrol insects across state or provincial borders; in Montana, permits can be obtained from the Montana Department of Agriculture.
Specifically to
Aphthona nigriscutis, adult beetles are the life stage to transfer and introduce. Adults can be obtained by sweep-netting at sites with established beetle populations. These can be stored for several days in cardboard containers with leafy spurge leaves if kept cool, and exercised and fed periodically under warmer conditions. Overwintering larvae can be dug from frozen host plant roots and soil material, and kept frozen until several weeks before adults are desired, at which time samples are removed from cold storage and allowed to warm to ambient or room temperature, thereby permiting larvae to develop and become adults. Adults should be sprinkled directly on leafy spurge plants (Rees et al. 1996; Lajeunesse et al. 1997)
Melissa Maggio-Kassner is the coordinator for the Montana Biological Weed Control Project. She can be reached at (406) 258-4223 or
mmaggio@missoulaeduplace.orgUseful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts WebpageMontana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesUseful Links:Central and Eastern Montana Invasive Species TeamMontana Invasive Species websiteAquatic Invasive SpeciesStewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Butler, J.L., Parker, M.S., Murphy, J.T. 2006. Efficacy of flea beetle control of Leafy Spurge in Montana and South Dakota. Rangeland Ecology and Management. 59:453-461.
Butler, J.L., Parker, M.S., Murphy, J.T. 2006. Efficacy of flea beetle control of Leafy Spurge in Montana and South Dakota. Rangeland Ecology and Management. 59:453-461. Fellows, D.O. and W.E. Newton. 1999. Prescribed fire effects on biological control of Leafy Spurge. Journal of Range Management. 52:489-493.
Fellows, D.O. and W.E. Newton. 1999. Prescribed fire effects on biological control of Leafy Spurge. Journal of Range Management. 52:489-493. Gassmann, A., D. Schroeder, E. Maw, and G. Sommer. 1996. Biology ecology and host specificity of European Aphthona spp. (Coleoptera, Chrysomelidae) used as biocontrol agents for Leafy Spurge, Euphorbia esula (Euphorbiaceae), in North America. Biological Control. 6:105-113.
Gassmann, A., D. Schroeder, E. Maw, and G. Sommer. 1996. Biology ecology and host specificity of European Aphthona spp. (Coleoptera, Chrysomelidae) used as biocontrol agents for Leafy Spurge, Euphorbia esula (Euphorbiaceae), in North America. Biological Control. 6:105-113. Hansen, R.W., R.D. Richard, P.E. Parker, and L.E. Wendell. 1997. Distribution of biological control agents of leafy spurge (Euphorbia esula L.) in the United States: 1988-1996. Biological Control 10:129-142.
Hansen, R.W., R.D. Richard, P.E. Parker, and L.E. Wendell. 1997. Distribution of biological control agents of leafy spurge (Euphorbia esula L.) in the United States: 1988-1996. Biological Control 10:129-142. Jackson, J.J. 1997. Biology of Aphthona nigriscutis (Coleoptera: Chrysomelidae) in the laboratory. Annals of the Entomological Society of America. 90:433-437.
Jackson, J.J. 1997. Biology of Aphthona nigriscutis (Coleoptera: Chrysomelidae) in the laboratory. Annals of the Entomological Society of America. 90:433-437. Jacobs, J.S., R.L. Sheley, N.R. Spencer, and G. Anderson. 2001. Relationships among edaphic, climatic, and vegetation conditions at release sites and Aphthona nigriscutis population density. Biological Control. 22:46-50.
Jacobs, J.S., R.L. Sheley, N.R. Spencer, and G. Anderson. 2001. Relationships among edaphic, climatic, and vegetation conditions at release sites and Aphthona nigriscutis population density. Biological Control. 22:46-50. Jonsen, I.D., R.S. Bourchier, and J.Roland. 2001. The influence of matrix habitat on Aphthona flea beetle immigration to leafy spurge patches. Oecologia. 127:287-294.
Jonsen, I.D., R.S. Bourchier, and J.Roland. 2001. The influence of matrix habitat on Aphthona flea beetle immigration to leafy spurge patches. Oecologia. 127:287-294. Lajeunesse, S., R. Sheley, R. Lym, D. Cooksey, C. Duncan, J. Lacey, N. Rees, and M. Ferrell. 1997. Leafy spurge: biology, ecology and management. Bozeman, MT: Montana State University Extension Service Bulletin EB-134. 25 p.
Lajeunesse, S., R. Sheley, R. Lym, D. Cooksey, C. Duncan, J. Lacey, N. Rees, and M. Ferrell. 1997. Leafy spurge: biology, ecology and management. Bozeman, MT: Montana State University Extension Service Bulletin EB-134. 25 p. Larson, D.L. and J.B. Grace. 2004. Temporal dynamics of leafy spurge (Euphorbia esula) and two species of flea beetles (Aphthona spp.) used as biological control agents. Biological Control. 29:207-214.
Larson, D.L. and J.B. Grace. 2004. Temporal dynamics of leafy spurge (Euphorbia esula) and two species of flea beetles (Aphthona spp.) used as biological control agents. Biological Control. 29:207-214. LeSage, L. and P. Paquin. 1996. Identification keys for Aphthona flea beetles (Coleoptera: Chrysomelidae) introduced in Canada for the control of spurge (Euphorbiaspp., Euphorbiaceae, in North America. Canadian Entomologist. 128:593-603.
LeSage, L. and P. Paquin. 1996. Identification keys for Aphthona flea beetles (Coleoptera: Chrysomelidae) introduced in Canada for the control of spurge (Euphorbiaspp., Euphorbiaceae, in North America. Canadian Entomologist. 128:593-603. Lym, R.G. 2005. Integration of biological control agents with other weed management technologies: successes from the leafy spurge (Euphorbia esula) IPM program. Biological Control 35:366-375.
Lym, R.G. 2005. Integration of biological control agents with other weed management technologies: successes from the leafy spurge (Euphorbia esula) IPM program. Biological Control 35:366-375. Lym, R.G. and J.A. Nelson. 2000. Biological control of Leary Spurge (Euphorbia edula) with Aphthona spp. along railroad right-of-ways. Weed Technology. 14:642-646.
Lym, R.G. and J.A. Nelson. 2000. Biological control of Leary Spurge (Euphorbia edula) with Aphthona spp. along railroad right-of-ways. Weed Technology. 14:642-646. Rees, N.E., R.W. Pemberton, N.R. Spencer, P.C. Quimby, Jr., and R.M. Nowierski. 1996. The spurges pp. 155-186 In: N.E. Rees, P.C. Quimby, Jr., G.L. Piper, E.M. Coombs, C.E. Turner, N.R. Spencer, and L.V. Knutson (eds). Western Society of Weed Science. Bozeman, MT: Montana State University.
Rees, N.E., R.W. Pemberton, N.R. Spencer, P.C. Quimby, Jr., and R.M. Nowierski. 1996. The spurges pp. 155-186 In: N.E. Rees, P.C. Quimby, Jr., G.L. Piper, E.M. Coombs, C.E. Turner, N.R. Spencer, and L.V. Knutson (eds). Western Society of Weed Science. Bozeman, MT: Montana State University. Skinner, L.C., D.W. Ragsdale, R.W. Hansen, M.A. Chandler, and G. Spoden. 2006. Phenology of first and peak emergence of Aphthona lacertosa, and A. nigriscutis: two flea beetles introduced for biological control of Leafy Spurge, Euphorbia esula L. Biological Control. 37:382-391.
Skinner, L.C., D.W. Ragsdale, R.W. Hansen, M.A. Chandler, and G. Spoden. 2006. Phenology of first and peak emergence of Aphthona lacertosa, and A. nigriscutis: two flea beetles introduced for biological control of Leafy Spurge, Euphorbia esula L. Biological Control. 37:382-391. Skinner, L.C., D.W. Ragsdale, R.W. Hansen, M.A. Chandler, and R.D. Moon. 2004. Temperature-dependent development of overwintering Aphthona lacertosa and A. nigriscutis (Coleoptera: Chrysomelidae): two flea beetles introduced for the biological control of Leafy Spurge, Euphorbia esula. Environmental Entomology. 33:147-154.
Skinner, L.C., D.W. Ragsdale, R.W. Hansen, M.A. Chandler, and R.D. Moon. 2004. Temperature-dependent development of overwintering Aphthona lacertosa and A. nigriscutis (Coleoptera: Chrysomelidae): two flea beetles introduced for the biological control of Leafy Spurge, Euphorbia esula. Environmental Entomology. 33:147-154. Wacker, S.D. and J.L. Butler. 2006. Potential impact of two Aphthona spp. On a native, nontarget Euphorbia species. Rangeland Ecology and Management. 59:468-474.
Wacker, S.D. and J.L. Butler. 2006. Potential impact of two Aphthona spp. On a native, nontarget Euphorbia species. Rangeland Ecology and Management. 59:468-474.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Wiman, N.G. 2001. Dynamics of leafy spurge (Euphorbia esula L.) infested plant communities influenced by flea beetles in the Aphthona complex (Colepotera: Chrysomelidae). M.Sc. Thesis. Bozeman, MT: Montana State University. 148 p.
Wiman, N.G. 2001. Dynamics of leafy spurge (Euphorbia esula L.) infested plant communities influenced by flea beetles in the Aphthona complex (Colepotera: Chrysomelidae). M.Sc. Thesis. Bozeman, MT: Montana State University. 148 p.
- Web Search Engines for Articles on "Black Dot Leafy Spurge Flea Beetle"
- Additional Sources of Information Related to "Insects"





