View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
False Mermaidweed - Floerkea proserpinacoides
Native Species
Global Rank:
G5
State Rank:
S3S4
(see State Rank Reason below)
C-value:
4
Agency Status
USFWS:
USFS:
BLM:
State Rank Reason (see State Rank above)
Floerkea proserpinacoides occurs from the valleys to the montane zones in portions of western Montana. Its habitat of shady, moist to wet soils within open forests, meadows, and along streams may represent specialized habitat. However, populations can be abundant and occur in both undisturbed and disturbed settings. It is unclear if Floerkea proserpinacoides thrives with some level of disturbance or prefers nutrient-enriched microsites. Monitoring and surveying that brings forth information on locations, population sizes, habitat, responses to disturbance, and threats is needed to better refine its status in Montana.
- Details on Status Ranking and Review
Range Extent
ScoreF - 20,000-200,000 sq km (~8,000-80,000 sq mi)
Comment41,304 square kilometers
Area of Occupancy
ScoreD - 6-25 4-km2 grid cells
CommentPlant occurs in 25 of the 30,590 4x4 square-kilometer grid cells that cover Montana.
Number of Populations
ScoreC - 21 - 80
Comment29 observations
Number of Occurrences or Percent Area with Good Viability / Ecological Integrity
ScoreC - Few (4-12) occurrences with excellent or good viability or ecological integrity
Comment6 observations assumed to have good viability based on descriptors of 'huge population', 'common', or 'locally common'.
Environmental Specificity
ScoreC - Moderate. Generalist or community with some key requirements scarce
Threats
ScoreD - Low
General Description
PLANTS: Fleshy, glabrous, fibrous-rooted annuals. Stems are prostrate to ascending, 5–25 cm long. Source: Lesica et al. 2012.
LEAVES: Pinnately-divided leaves with a long petiole and alternately arranged along the stem. Leaves have 3-7 leaflets. Leaflets are narrowly elliptic, 5-20 mm long. Source: Lesica et al. 2012.
INFLORESCENCE: Flowers are inconspicuous - largely solitary, borne on long pedicels in the axils of leaves. Flowers nod or are upright. Flowers perfect, regular, hypogenous, 3-merous; sepals united at the base, 2–3 mm long; petals separate, white, spatulate, 1–2 mm long; stamens 3 to 6; ovary with 2 or 3 carpels; style 1. Fruit 2 or 3 bumpy, globose nuts, 1–3 mm long, united at the base, partially enclosed by the calyx. Source: Lesica et al. 2012.
Phenology
A spring annual. In Wisconsin flowering occurs continuously from about mid-April to early June (Smith 1983). On the Beaverhead-Deerlodge National Forest in Montana plants are often found in June, and not any later (Wallace-Senft personal communication).
Species Range
Montana Range
Range Descriptions

 Native
Native
Range Comments
Throughout most of temperate North America (Lesica et al. 2012).
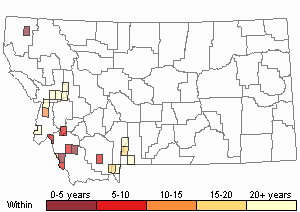
Observations in Montana Natural Heritage Program Database
Number of Observations: 30
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
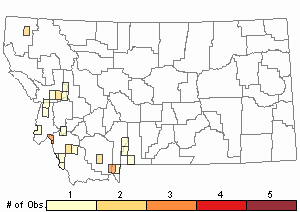
Recency
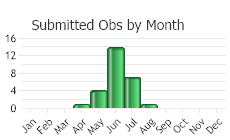
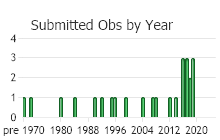
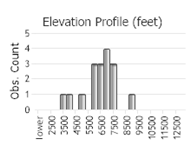 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Wet, shady soil of open forest, meadows, and often along streams within the valleys to montane zones (Lesica et al. 2012).
On the Beaverhead-Deerlodge National Forest in Montana, Floerkea proserpinacoides has been observed to associate with Nemophila brevifolia (Wallace-Senft personal communication). Both species occur in the transitional area between an inundated stream or wetland and its bank. Nemophila usually grows on slightly drier ground than Floerkea which often grows where it is not quite inundated. Floerkea plants are usually found mixed with and under a dense canopy of other graminoids and forb cover, such that other plants must be brushed away to clearly see it. Floerkea seems to occur where micro-habitat is transitional, such as in wetlands with cow hoof prints; at edges of roads where a steep slope, gravel, and water combine; at culverts; seeps or springs with some livestock presence; and where significant land slumping has occurred (Wallace-Senft personal communication). These micro-habitats also appear to be nutrient-rich areas whether from livestock or minerals.
Ecology
PREDATION
A host-specific fungal parasite, Peronospora floerkeae Kell., is known to infect False Mermaid plants and reduce their fecundity (Smith 1983).
Demographic studies in Wisconsin led to circumstantial evidence that the White-footed Deer Mouse (Peromyscus leucopus), Eastern Chipmunk (Tamias striatus), and Short-tailed Shrew (Blarina brevicauda) which are common in the study area were not attracted to seeds produced by False Mermaid. Further invertebrate predation was only observed once, seen as a bored hole in the seed, in a 5-year study (Smith 1983c).
Low predation pressure on False Mermaid plants could be attributed to their flavonoid substances (Smith 1983c). Several types of flavonol glycosides have been measured at high concentrations in False Mermaid plants (Parker and Bohm 1979). Another study found that ingesting flvonol glycosides can inhibit enzyme function in insects at physiological concentrations (Wagner 1979), and the presence of several different glycosides in a plant helps inhibit feeding. Flavonoids that occur in False Mermaid have been shown to affect cell membrane structure and calcium transport in rat cells (Fewtrell and Gomperts 1977).
POPULATION DYNAMICS
In forests of the mid-west the distribution of False Mermaid is often patchy and populations are usually discrete, appear clone-like, and vary is size from less than 1 meter to more than 15 meters (Smith 1983a). Demography studies carried out by Smith (1983a) examined the interactions among False Mermaid plant and population growth and light, nutrient and water availability in Wisconsin. Smith found that as a population increased in density, the average plant size and life-span decreased. In the spring time, water and nutrient availability remained high in the soils beneath maple (Acer spp.) forests and weren’t found to limit plant growth. However, as spring develops the deciduous canopy closes in and light to the forest floor is reduced. Individuals growing in dense populations also become self-shaded. Smith concluded that self-shading augmented with decreasing light through the canopy caused lower productivity, smaller plant size, and an earlier death in high density populations. Node production was most affected by density, in that fewer nodes were produced in plants growing in dense populations. However, populations did not appear to undergo self-thinning. Density-dependent plasticity in seed production and mortality at life-cycle stages prior to the emergence of seedlings above the litter prevents population density from being sufficiently high where self-thinning becomes important.
Once a population is initiated, individual fecundity becomes density dependent in the local area within a few years (Smith 1983b and 1983c). It is likely that very large populations are composed of populations that initiated at different times and became merged as they grew.
False Mermaid exploits open habitat, but it also grows in later-successional communities, such as forests dominated by Sugar maple (Acer saccharum), a shade-tolerant, climax species (Smith 1983b). Smith found that during the 5-year demography study (1976 to 1981) False Mermaid consistently senesced at the same time each spring despite that the window for seedling emergence varied by 3-weeks during (1983). Smith also found that flower production and seed set decreased earlier in high-density populations. Lower density populations had a longer time of favorable conditions for seed production when compare to dense populations. Fecundity, the number of seeds produced per plant, was more variable from year to year for low density populations. When the spring growing season is long, the differences in fecundity between low and high density population will be larger.
In deciduous forests, soil chemistry is strongly affected by tree species composition (Smith 1983c). In Wisconsin, False Mermaid commonly grows under the trees of Sugar Maple, but rarely grows under Red Oak (Quercus rubra). Attributes of Red Oak create less favorable habitat for False Mermaid. Under oak canopies the soil tends to be more acidic and have a lower nutrient availability. Oak leaves are harder and persist longer creating deeper litter.
Reproductive Characteristics
AUTOGAMY and SEED PRODUCTION
False Mermaid plants produce single-seeded nutlets. Reproduction is autogamous, where seeds are self-fertilized by the parent plant. Demography studies by Smith (1983b) support the theory that autogamy is often advantageous when pollinators are scare and unpredictable. In Wisconsin False Mermaid plants often flower when temperatures are too low for bees. While increased temperatures bring higher bee activity, the deciduous canopy begins to close and with it, False Mermaid growth and seed production decline. Thus, under these conditions autogamy will increase the chance that pollination can occur in early spring. Smith found that False Mermaid flowers that developed early in the growing season had a higher probability to set their seeds and to produce more seeds per fruit.
False Mermaid is considered to exhibit low fecundity, yet in comparison to other forest-floor plants seed weight is high (Smith 1983b). It is assumed that these plants require a large seed in order to have enough stored food to ensure that seedlings establish in the deep litter layer. It must be assumed that the advantages of having a large seed outweigh the disadvantages of producing more seeds.
Mokhtar and Houle studied the relationship of plant senescence and fruit maturation with False Mermaid (2005). Fruits (nutlets) removed from plants grown in full light and in shade increased their stem and leaf biomass when compared with grown plants with fruit. Fruit maturation occurs after leaves begin to senesce and roots begin to disintegrate. The authors suggested that carbon and other nutrients that are mostly accumulated in the leaves and stems are translocated to the maturing fruits and represent a strategy to optimize resources. Further they found that decreasing light conditions do not seem immediately responsible for plant senescence, but that fruit maturation does.
In natural populations in Wisconsin, seed yield often exceeds 5000 seeds per square meter (Smith 1983b). Seed dormancy appears to be short-lived and the year to year seed bank overlaps little (Smith 1983c). Smith found that from 1 to 12 percent of seeds survived to the next growing season.
In Wisconsin, Smith (1983b) studied the effect of plant density on the number of seeds produced by a False Mermaid plant (fecundity). Seed production per unit area leveled off with increasing plant density (Smith 1983b). In populations that were already dense, seed yield was independent of population density.
LIFE CYCLE [Adapted from Smith 1983a and 1983c]
In Wisconsin, False Mermaid is a short-lived (60-70 days) spring ephemeral. Fully-opened flowers (anthesis) contain three ovules, yet typically produce a single, large seed (rarely three seeds). Seeds germinate in winter, but emergence above the leaf-litter varies from late March to mid-April, and depends upon spring temperature patterns. Seedlings have small shoots that cannot push up the leaf litter, rather it grows through holes found within the litter, and uses stored food reserves in the seed and on the petiole (not shoot) etiolation for successful emergence. The cotyledons develop below the ground. Once the plant emerges its growth and development is rapid. Plants grow upright until they are about 10-15 cm and then they grow more prostrate. The first two nodes of young stems develop a trifoliate leaf. The remaining nodes will develop pinnately-divided leaves of 5-7 leaftlets. The roots remain confined to the upper 2 cm of the humus layer of the soil. With development the lowest three nodes may later produce branches, while the upper notes tend to produce axillary flowers. Flowering occurs from mid-april to early June. In deciduous forests, growth decreases in mid-May as the tree canopy closes. Plants senesce in June.
From demography sites Smith studied seed dispersal by False Mermaid (1983c). Plants produce single-seeded nutlets that have no obvious dispersal mechanism (Smith 1983c). Although the surface of the nutlet is tuberculate they lack an appendage that would facilitate dispersal by wind or animals. Seeds are soft and it is suspected that they would not survive if ingested by an animal. Plants are often prostrate by the time seeds shed, thus, it is assumed that the plant’s shoot is the primary dispersal agent.
Management
A severe ground fire can destroy the seed bank of False Mermaid, causing local extinction (Smith 1983c).
False Mermaid is absent where forest leaf litter accumulates too deeply (Smith 1983c). Trees that fall over existing populations could reduce local populations if litter depth increased significantly (Smith 1983c).
Stewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Houle, Gilles. 2002. The Advantage of Early Flowering in the Spring Ephemeral Annual Plant Floerkea proserpinacoides. The New Phytologist, Volume 154(Number 3), pp. 689-694.
Houle, Gilles. 2002. The Advantage of Early Flowering in the Spring Ephemeral Annual Plant Floerkea proserpinacoides. The New Phytologist, Volume 154(Number 3), pp. 689-694. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Mokhtar, Ines Ben, and Gilles Houle. 2005. Fruit Maturation, Not Deteriorating Light Conditions, Is the Primary Cue to Senescence in a Spring Ephemeral Annual Plants: Floerkea proserpinacoides (Limnanthaceae). American Journal of Botany, Volume 92(3), pp. 438-442.
Mokhtar, Ines Ben, and Gilles Houle. 2005. Fruit Maturation, Not Deteriorating Light Conditions, Is the Primary Cue to Senescence in a Spring Ephemeral Annual Plants: Floerkea proserpinacoides (Limnanthaceae). American Journal of Botany, Volume 92(3), pp. 438-442. Parker, W., and B. Bohm. 1979. Flavonoids and Taxonomy of the Limnanthaceae. American Journal of Botany, 66, pp. 191-197.
Parker, W., and B. Bohm. 1979. Flavonoids and Taxonomy of the Limnanthaceae. American Journal of Botany, 66, pp. 191-197. Smith, Brent. 1983a. Demography of Floerkea proserpinacoides, A Forest-Floor Annual: I. Density-Dependent Growth and Mortality. Journal of Ecology, Volume 71(2), pp. 391-404.
Smith, Brent. 1983a. Demography of Floerkea proserpinacoides, A Forest-Floor Annual: I. Density-Dependent Growth and Mortality. Journal of Ecology, Volume 71(2), pp. 391-404. Smith, Brent. 1983b. Demography of Floerkea proserpinacoides, A Forest-Floor Annual: II. Density-Dependent Reproduction. Journal of Ecology, Volume 71(2), pp. 405-412.
Smith, Brent. 1983b. Demography of Floerkea proserpinacoides, A Forest-Floor Annual: II. Density-Dependent Reproduction. Journal of Ecology, Volume 71(2), pp. 405-412. Smith, Brent. 1983c. Demography of Floerkea proserpinacoides, A Forest-Floor Annual: III. Dynamics of seed and Seedling Populations. Journal of Ecology, Volume 71(2), pp. 413-425.
Smith, Brent. 1983c. Demography of Floerkea proserpinacoides, A Forest-Floor Annual: III. Dynamics of seed and Seedling Populations. Journal of Ecology, Volume 71(2), pp. 413-425. Wagner, H. 1979. Phenolic compounds in plants of pharmaceutical interest. Recent Advances in Phytochemistry, 12, pp. 589-616.
Wagner, H. 1979. Phenolic compounds in plants of pharmaceutical interest. Recent Advances in Phytochemistry, 12, pp. 589-616.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Fewtrell, C., and B. Gomperts. 1977. Effect of Flavone Inhibitors of Transport ATPase on Histamine Secretion From Rat Mast Cells. Nature, London, 265, pp. 635-636
Fewtrell, C., and B. Gomperts. 1977. Effect of Flavone Inhibitors of Transport ATPase on Histamine Secretion From Rat Mast Cells. Nature, London, 265, pp. 635-636 Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
- Web Search Engines for Articles on "False Mermaidweed"





