View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Rush Skeletonweed - Chondrilla juncea
Other Names:
Hog Bite
State Rank Reason (see State Rank above)
Chondrilla juncea is a plant native to the Mediterranean region of Europe, North Africa, and Asia minor (FNA 2006). Chondrilla juncea was first detected in Sanders County, Montana in 1987. In 2021 the MTNHP Botanist conducted a state-wide status review. Although its State distribution has expanded, control efforts are showing some successes. A conservation status rank is not applicable (SNA) because the plant is an exotic (non-native) in Montana that is not a suitable target for conservation activities.
NOTE: It is important to document new occurrences by providing nice quality plant specimens to one of our State herbaria (University of Montana, Montana State University, or Montana State University-Billings). Herbarium specimens allow identifications to be confirmed, provide a central location for educating and information sharing, are a source for genetic, morphological, and ecological studies, and are the basis for which Montana's floras are written.
General Description
PLANTS: A taprooted perennial forb with milky sap. Stems are ascending to erect, branched, and 25–100 cm tall. Herbage is hirsute to glabrate.
LEAVES: Leaves occur as a rosette (basal) and along the stem (cauline). Basal leaves are petiolate, oblong, pinnately lobed with sharply dentate margins, 5–13 cm long, and deciduous. The lobes of the basal leaves point backwards, toward the leaf base. Cauline leaves are sparse or inconspicuous, alternately arranged, linear-oblong, and 2–10 cm long.
INFLORESCENCE: Yellow flower heads occur solitary or in groups of 2-5 in the axils of the branches and at the tips of branches.
Chondrilla is the name used by Pedanius Dioscorides, a Greek physician, for a plant that exudes milky juice or gum (FNA 2006). Its common name may refer to its stems which are essentially leafless (or have inconspicuous leaves) and when the rosette leaves wither, the plant takes on a skeleton-like form (Jacobs and Goodwin 2015).
Phenology
Flowering July through October (FNA 2006).
Diagnostic Characteristics
Whether by common names, scientific name, or their initial appearance these plants can be confused with one another leading to mis-identifications or computer coding errors. Here is how to differentiate them:
Rush skeletonweed -
Chondrilla juncea, exotic and Noxious:
* In Aster Family; yellow flowerheads occur at the tips of stems or in the axils of leaves. About 7-15 florets per flowerhead.
* Achenes (fruits) have a beak 5-6mm long.
* Stems, leaves, and inflorescence have milky sap.
* Upper stems are nearly hairless (glabrate). The lower 10-15 cm of stem has downward pointing hairs.
* Leaves on stem (cauline) are short and narrow, often sparse.
* Basal leaves form a rosette with downward pointing lobes, often whithering by flowering time.
* Plants are taprooted perennial forbs.
Rush Skeleton-plant -
Lygodesmia juncea, native:
* In Aster Family; pinkish flowerheads occur at the tips of stems. About 5 florets per flowerhead.
* Achenes are not beaked, but sometimes are fusiform.
* Plants lack milky sap.
* Plants lack hairs (glabrous).
* Cauline leaves are short and narrow, reduced to scales in the upper branches.
* Basal leaves are absent.
* Plants are rhizomatous perennial forbs.
Spiny Skeletonweed -
Pleiacanthus spinosus, native, SOC:
* In Aster Family; pinkish flowerheads occur solitary on upper branches. About 3 florets per flowerhead.
* Achenes are not beaked, but are tubular.
* Plants have a milky sap.
* Plants have hairs, glabrate to weakly tomentose.
* Stems are spine-tipped and branched with inconspicuous short and narrow leaves.
* Basal leaves are absent.
* Plants are taprooted with a brown-woolly branched crown.
* Found in south-central Montana.
Tall Tumble-mustard -
Sisymbrium altissimum, exotic:
* In Mustard Family; flowers with 4 (pale) yellow petals and 4 green to yellowish sepals.
* Stems are leafy with large pinnately lobed leaves.
* In fruit or during the winter stems appear leafless. Plants branch, becoming round.
* Fruit is a long and narrow silique that is equal in width to its stem (pedicel).
Immature Plants - Basal RosettesWhen immature,
Rush Skeletonweed rosettes can appear like
Common Dandelion (
Taraxacum officinale) rosettes. Both have pinnately-lobed basal leaves with lobe tips that point backward toward the leaf base and that exude a milky sap. Rush Skeletonweed basal leaves are stiff, have hairs, and usually whither by flowering time while Dandelion basal leaves are glabrous or hairy on the mid-veins and don't whither during flowering.
The basal leaves of
Sow-thistle (
Sonchus) are also pinnately lobed with prickly margins.
The lower leaves of
Rush Skeleton-plant (
Lygodesmia juncea) are simple and not in a rosette: 5-30(60) mm by 1-2(-4) mm, margins entire, tips acute, and blades without hairs (glabrous). The upper leaves are reduced to subulate scales.
Species Range
Range Comments
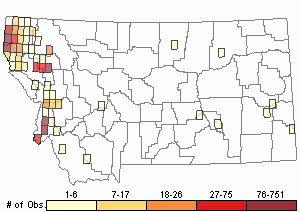
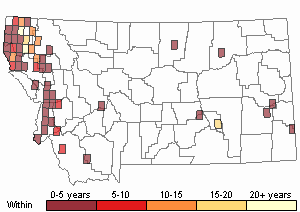
Based on a review of information conducted by the MTNHP Botanist in 2021, Chondrilla juncea was first reported in 1987 from a Christmas tree plantation in Sanders County, Montana. As of 2021 the herbarium record has validated this plant's occurrence in Lincoln, Sanders, Treasure, and Ravalli Counties of Montana. Observation data, both accurate and likely accurate in identification and/or mapping, have found additional sites Chondrilla juncea in Flathead, Lake, Missoula, Ravalli, and Beaverhead Counties. A 2004 infestation in Treasure County led to an extensive multi-agency control effort and in 2006 and 2007 plants were not observed (Cramer personal communication). Despite the prevalence of observations and an expansion in distribution, the Chondrilla juncea task force along with others are showing successes at controlling its spread.
For maps and other distributional information on non-native species see:
Nonindigenous Aquatic Species Database from the U.S. Geological Survey
Invasive Species Habitat Tool (INHABIT) from the U.S. Geological Survey
Invasive Species Compendium from the Centre for Agriculture and Bioscience International (CABI)
EDDMapS Species Information EDDMapS Species Information
Observations in Montana Natural Heritage Program Database
Number of Observations: 4995
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
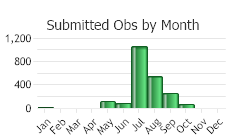
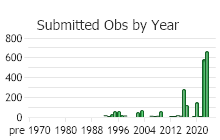
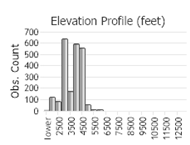 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Habitat
Roadsides, rangelands, fields, waste places, and other disturbed ground (FNA 2006). Grows within valleys in Montana (Lesica et al. 2012).
Ecology
Rush Skeletonweed grows in very dry to very wet environments and can invade both crop and rangelands (Sheley et al. in Sheley and Petroff 1999). Optimal conditions for growth and reproduction are sites with cool winters and warm summers that have winter and spring rainfall, but lack severe drought. Summer temperatures of at least 59 degrees Fahrenheit are necessary for flower and seed development. Drought will reduce seed production. However, it is considered drought-tolerant in Australia as well as palatable and nutritious for sheep (Sheley et al. in Sheley and Petroff 1999).
Rush Skeletonweed apparently seldom invades healthy native vegetation, but does colonize disturbed areas such as roadways, waste areas, and sites weakened by drought or improper grazing management (Sheley et al. in Sheley and Petroff 1999). Plants also prefer well-drained, sandy-textured or rocky soils. Habitat types that are susceptible to invasion by Rush Skeleton weed include: Big Sagebrush/Needle-and-Thread Grass (Artemisia tridentata/Stipa comata), Bluebunch Wheatgrass/Sandberg’s Bluegrass (Elymus spicatus/Poa secunda), and Bitterbrush/Bluebunch Wheatgrass (Purshia tridentata/E. spicatus).
In Australia Rush Skeletonweed is a source of pollen for honeybees (Sheley et al. in Sheley and Petroff 1999).
Reproductive Characteristics
Chondrilla juncea only produces seeds through asexual reproduction (FNA 2006). Specifically, seeds develop by a parthenogenetic process where there is development of an egg without fertilization (FNA 2006). Despite this the species is highly variable in morphology and biochemical traits (FNA 2006). In Australia more than 300 morphological forms have been recognized of which 3 are found in the United States (Sheley et al. in Sheley and Petroff 1999). In the U.S. these forms (or genotypes) differ in having narrow, intermediate, and broad rosette leaves (Sheley et al. in Sheley and Petroff 1999). Plants also vary in their inflorescence morphology, fruits, potential for regrowth from roots, and susceptibility to biological or chemical controls (Sheley et al. in Sheley and Petroff 1999).
FLOWERS [Adapted from Sheley et al. in Sheley and Petroff 1999; Lesica et al. 2012]
Heads ligulate, short-peduncled in axils of upper reduced leaves. Involucres 8–12 mm high, sparsely tomentose, subtended by small bracts; phyllaries 5 to 9, linear-lanceolate in 1 series. Ray flowers perfect, yellow, 7 to 15; ligules 4–6 mm long. Pappus is of capillary bristles, which helps the seed float in wind.
FRUITS [Adapted from Sheley et al. in Sheley and Petroff 1999; Lesica et al. 2012]
Achenes (fruits) are cylindric, glabrous, ribbed, and 3–4 mm long with a 5–6 mm beak (Lesica et al. 2012). Seeds apparently have no dormancy and can germinate within 24 hours under optimal conditions (59 to 86 degrees Fahrenheit). Seeds that are not buried remain viable for 6 to 18 months. Seeds buried in the soil may take 1-2 years before they germinate. A single plant can produce up to 10,000 seeds.
ROOTS [Adapted from Sheley et al. in Sheley and Petroff 1999; Lesica et al. 2012]
Plants produce taproots that can grow to 2.4 meters deep with few lateral roots. Adventitious buds are where new shoots grow and allow the plant to be perennial; buds develop at the top of the taproot and on major lateral roots. Roots that are severed above about four feet will grow new shoots. Even tiny taproots that are severed can produce new plants if soil is moist. In general the ability for new shoots to emerge increases with root size and decreases with soil depth.
LIFE CYCLE [Adapted from Sheley et al. in Sheley and Petroff 1999; Jacobs and Goodwin 2015]
In the fall seeds germinate into seedlings and established plants re-grow from perennial taproots. Seedlings require continuous rainfall for 3-6 weeks in order to establish. Plants overwinter as rosettes. Plants can resume growth at any time when temperatures are above freezing; however, significant growth usually begins in March to April. In late spring, a single stem arises from the rosette, which soon branches. In early summer, flowering and seed development begin. Flowerheads (also called capitula) open in the morning and close before sunset. In hot, dry conditions the flowerheads open for a short duration and seeds form without pollination. Seeds mature about 9-15 days after flowers open. As seeds are dispersed by the wind, plants dieback. Upon fall rains germination and re-growth begins the cycle again.
Management
See
Noxious Weed Education VideoSmaller infestations can be eradicated or prevented from establishing, while larger infestations will require an integrated management strategy involving cultural, chemical, and biological controls Sheley et al. in Sheley and Petroff 1999. For existing infestations, an eradication plan should be designed and implemented. An effective plan should map infestation boundaries and include details on control treatments, control schedule, revegetation plans, follow-up monitoring, and costs.
Yearly surveys are key to controlling or eradicating infestations. In Lincoln County, infestations are often small and control measures are showing their effectiveness. However, sites thought to be eradicated need to be surveyed annually or every few years because plants can be found 6 years, even 12 years, after their detection (Andreasen personal communication).
PREVENTION [Adapted from Sheley et al.
in Sheley and Petroff 1999]
Strategies that negatively impact seed production and dispersal will prevent invasions. Seeds are dispersed primarily by wind, but also by water, trains, vehicles, and farm equipment. Vehicles and other motorized equipment should not pass through infestations particularly if in seed. The undercarriage of vehicles should be thoroughly washed before leaving infested areas. Livestock should not be moved from infested to uninfested areas. Plants along roads, waterways, and railways must be controlled to reduce spread. Systematic surveys can detect new infestations and reduce control efforts over time.
GRAZING MANAGEMENT [Adapted from Sheley et al.
in Sheley and Petroff 1999]
Proper grazing management should strive to maintain healthy rangeland and pasture vegetation which will compete against Rush Skeletonweed encroachment. To help achieve proper forage utilization, it is recommended to alter the season/timing of use, alter stock rates and rotate use to allow plants to recover before being re-grazed, and to promote plant litter accumulation.
Livestock that have grazed where Rush Skeletonweed occurs should be moved to a holding pasture or area for 10-14 days before moving into weed-free areas.
MECHANICAL and PHYSICAL CONTROLS [Adapted from Sheley et al.
in Sheley and Petroff 1999]
Hand-pulling can be effective for small infestations, particularly when soil is moist or where sandy. It may be necessary to pull plants 2-3 times per year for 6-10 years to remove plants that resprout from severed roots or that emerge from buried seed. Plants can be burned to ensure that seed and root material dies. Alternatively, plants should be bagged and desiccated or rotted before disposing.
Burning is not an effective treatment because it stimulates shoot growth from roots and seed germination. Prescribed burns should not be conducted in or near to Rush Skeletonweed infestations.
Mowing may reduce seed production, particularly in dry years, but overall is not an effective treatment for this long-lived perennial forb.
Cultivation is not an effective treatment because severing roots could lead to new growth.
Irrigation by itself is not an effective treatment because it stimulates seed germination and seedling growth. When used with fertilizers, forag harvest, grazing management, and competitive vegetation, it can reduce infestations or preent re-establishment.
CHEMICAL CONTROL [Adapted from Sheley et al.
in Sheley and Petroff 1999; Jacobs and Goodwin 2015]
Rush Skeletonweed is difficult to control using herbicides, because chemicals poorly translocate through the extensive root system and the small amount of leaf surface doesn’t allow for adequate retention and absorption. Effective herbicide control depends upon site conditions, requires the use of a silicone surfactant or water conditioning agent, and an aggressive re-application plan. This may impart be because herbicides poorly translocate through the extensive root system. Herbicides have been most effective when used on plants infected by a biocontrol organism.
Picloram applied at 2 pounds active ingredient per acre (lb ai/ac) to rosettes has been a standard for Rush Skeleton. However, Picloram applied at 1 quart product per acre alone or mixed with 2,4-D at 1 quarter per acre provide the best control when applied to rosettes in the fall. Follow-up treatment the next fall will likely be necessary.
Clopyralid,
Aminopyralid, and
Dicamba tanslocate herbicides to the root as well. Consultation with local Extension agents is recommended.
REVEGETATION [Adapted from Sheley et al.
in Sheley and Petroff 1999]
Rush Skeletonweed encroaches where land is disturbed. Maintaining healthy vegetation will help compete against Rush Skeletonweed. Planting competitive legumes, such as alfalfa (Medicago sativa), increases soil nitrogen which hinders Rush Skeletonweed in crop-pasture rotations. Dense stands of legumes also compete well for soil moisture and create shade which reduces Rush Skeletonweed. Rush Skeletonweed does not grow well in shaded conditions.
In general, nitrogen increased the size of Rush Skeleton plants, but decreased their density. Nitrogen boosts the competive edge of desirable vegetation, which then results in lower weed density.
BIOLOGICAL CONTROL [Adapted from Sheley et al.
in Sheley and Petroff 1999; Jacobs and Goodwin 2015]
Three biological control agents have been approved and released, but their effectiveness has been site specific.
The
Skeleton Root Moth (
Bradyrrhoa gilveolella) was introduced into Idaho in 2002, but by 2009 it status is uncertain.
The
Rush Skeletonweed Gall Midge (
Cystiphora schmidti) was introduced in California in 1975. the midge damages rosettes and flowering stems and reduces seed production. Its populations are prevalent in California, Idaho, and Oregon.
The
Rush Skeletonweed Rust Fungus (
Puccinia chondrillina) is the first exotic plant pathogen to be introduced asa classical biological control agent in North America. The rust creates postules in the leaves and stems which reduces the plant’s ability to photosynthesize and dries out the leaves. Its effectiveness depends upon the plant’s genotype (refer to REPRODUCTIVE CHARACTERISTICS) and other site conditions.
Montana's Rush Skeletonweed Task Force is led by Mark Andreasen who can be contacted at: (406) 283-2420 or
lcweed@frontiernet.netUseful Links:Montana Invasive Species websiteMontana Biological Weed Control Coordination ProjectMontana Department of Agriculture - Noxious WeedsMontana Weed Control AssociationMontana Weed Control Association Contacts Webpage.
Montana Fish, Wildlife, and Parks - Noxious WeedsMontana State University Integrated Pest Management ExtensionWeed Publications at Montana State University Extension - MontGuidesStewardship Responsibility
Threats or Limiting Factors
Rush Skeletonweed competes strongly against crops for soil moisture and nitrogen, and the wiry stems interfere with harvesting (Sheley et al. in Sheley and Petroff 1999).
Rush Skeletonweed forms dense monocultures on disturbed land, and can replace native plants, and reduces forage production (Sheley et al. in Sheley and Petroff 1999).
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Flora of North America Editorial Committee. 2006. Flora of North America North of Mexico. Vol. 19. Magnoliophyta: Asteridae, part 6: Asteraceae, part 1. Oxford Univ. Press, New York. xxiv + 579 pp.
Flora of North America Editorial Committee. 2006. Flora of North America North of Mexico. Vol. 19. Magnoliophyta: Asteridae, part 6: Asteraceae, part 1. Oxford Univ. Press, New York. xxiv + 579 pp. Jacobs, Jim, and Kim Goodwin. 2009. Ecology and Management of Rush Skeletonweed (Chondrilla juncea L.). August. U.S. Department of Agriculture, Natural Resources and Conservation Service, Bozeman, Montana.
Jacobs, Jim, and Kim Goodwin. 2009. Ecology and Management of Rush Skeletonweed (Chondrilla juncea L.). August. U.S. Department of Agriculture, Natural Resources and Conservation Service, Bozeman, Montana. Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2012. Manual of Montana Vascular Plants. Fort Worth, TX: BRIT Press. viii + 771 p. Pipp, Andrea. 2021. Correspondence on Chondrilla juncea in Treasure County. July 29th-August 5th. Electronic mail correspondence between Andrea Pipp, MTNHP Botanist, Helena, MT and Jennifer Cramer, Treasure County Weed District Coordinator, Hysham, MT.
Pipp, Andrea. 2021. Correspondence on Chondrilla juncea in Treasure County. July 29th-August 5th. Electronic mail correspondence between Andrea Pipp, MTNHP Botanist, Helena, MT and Jennifer Cramer, Treasure County Weed District Coordinator, Hysham, MT. Pipp, Andrea. 2021. Correspondences on Chondrilla juncea in Lincoln County and Montana. July 27th to September 5th. Mark Andreasen, Task Force Coordination and Mapping, Lincoln County Weed District, Libby, MT and Andrea Pipp, Botanist, MTNHP, Helena, MT.
Pipp, Andrea. 2021. Correspondences on Chondrilla juncea in Lincoln County and Montana. July 27th to September 5th. Mark Andreasen, Task Force Coordination and Mapping, Lincoln County Weed District, Libby, MT and Andrea Pipp, Botanist, MTNHP, Helena, MT. Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
Sheley, Roger, and Janet Petroff. 1999. Biology and Management of Noxious Rangeland Weeds. Oregon State University Press, Corvallis, Oregon.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p.
Lesica, P., M.T. Lavin, and P.F. Stickney. 2022. Manual of Montana Vascular Plants, Second Edition. Fort Worth, TX: BRIT Press. viii + 779 p. Maier, C.A. 2010. Taxanomic studies in the metallic wood-boring beetle family (Coleoptera: Buprestidae: Sphenoptera and Chalcophora). M.Sc. Thesis. Bozeman, MT: Montana State University. 149 p.
Maier, C.A. 2010. Taxanomic studies in the metallic wood-boring beetle family (Coleoptera: Buprestidae: Sphenoptera and Chalcophora). M.Sc. Thesis. Bozeman, MT: Montana State University. 149 p. Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park.
Olliff, Tom, Roy Renkin, Craig McClure, Paul Miller, Dave Price, Dan Reinhart, and Jennifer Whipple. 2001. Managing A Complex Exotic Vegetation Program in Yellowstone National Park. Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p.
Quire, R.L. 2013. The sagebrush steppe of Montana and southeastern Idaho shows evidence of high native plant diversity, stability, and resistance to the detrimental effects of nonnative plant species. M.Sc. Thesis. Bozeman, MT: Montana State University. 124 p.
- Web Search Engines for Articles on "Rush Skeletonweed"





