View in other NatureServe Network Field Guides
NatureServe
Montana
Utah
Wyoming
Idaho
Wisconsin
British Columbia
South Carolina
Yukon
California
New York
Long-toed Salamander - Ambystoma macrodactylum
Native Species
Global Rank:
G5
State Rank:
S5
(see State Rank Reason below)
Agency Status
USFWS:
USFS:
BLM:
External Links
State Rank Reason (see State Rank above)
Species is common, widely distributed, and stable.
General Description
EGGS
Typically laid in ponds in small clusters of 9-81 (X = 23, SD = 14.5, N = 36 across 5 sites in northwest Montana). Each ovum is black or brown above, white to gray below, and surrounded by two jelly layers. Ovum diameters are approximately 2.5 mm (0.1 in), but total egg diameters, including the two jelly layers, are usually 12-17 mm (0.47-0.67 in) (Slater 1936).
LARVAE
Translucent, light tan, or black dorsally and laterally with black and gold flecks. White to
pinkish ventrally. Three pairs of external feathery gills emanate from the sides of the head with 9-13 gill rakers on their anterior surface (Russell and Bauer 2000). Snout-vent length (SVL) of 10-60 mm (0.4-2.4 in).
JUVENILES AND ADULTS
Fourth toe on the hind foot is elongate and longer than the sole of the foot. Incomplete or fully formed yellow, orangish, or reddish dorsal stripe may extend from the tip of the snout to the tip of the tail. Eyelids are the same color as the dorsal stripe. White flecking present on the lateral and ventral surfaces over a black lateral and pink ventral base color. 12-13 costal grooves are present. SVL of 25-80 mm (1-3.1 in) (Russell et al. 1996).
Diagnostic Characteristics
Adult Coeur d’Alene Salamanders (
Plethodon idahoensis) have nasolabial grooves and their toes are webbed and shorter than the soles of their feet. See sections on habitat use for differences in habitat used by Long-toed and Coeur d’Alene Salamanders. Western Tiger Salamander (
Ambystoma mavortium) eggs have 3 jelly layers and have total diameters less than 10 mm (0.4 in), including the jelly layers. Larval Western Tiger Salamanders have larger heads are usually olive green to silvery white in base color and have 15-25 gill rakers on the anterior surface of their gills (Russell and Bauer 2000). See sections on distribution for geographic areas of possible overlap for Long-toed and Western Tiger Salamanders.
Species Range
Montana Range
Range Descriptions
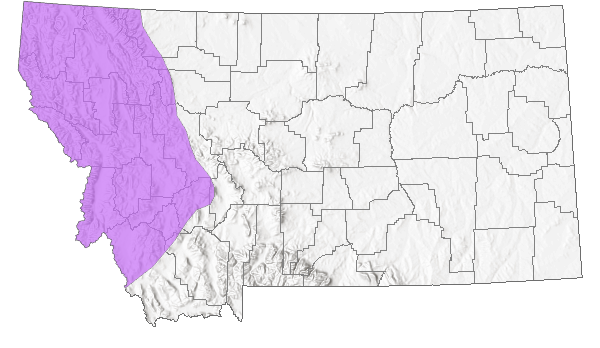
 Native
Native
Global Range

Estimated global breeding range area is: 1,812,717 km
2 (Approximately 5% in Montana)
See information about
 iNaturalist Geomodels
iNaturalist Geomodels
Range Comments
Five subspecies are recognized and range from central California through the Pacific Northwest
to southeast Alaska at elevations up to or above 2,700 m (8,859 ft) (Ferguson 1961; Petranka
1998). Only a single subspecies, the Northern Long-toed Salamander (A. m. krausei), occurs in Montana. Their known range in Montana extends west of the Rocky Mountain Front and the Missouri, Jefferson, and Beaverhead Rivers.
Maximum elevation: 2,774 m (9,100 ft), Beaverhead County K. Storrar (Werner et al. 2004).
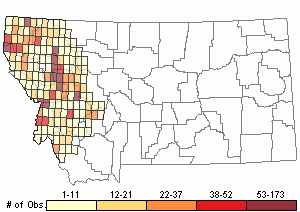
Observations in Montana Natural Heritage Program Database
Number of Observations: 2912
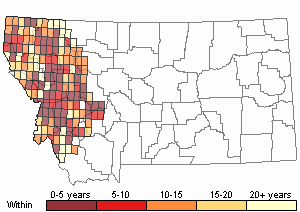
(Click on the following maps and charts to see full sized version)
Map Help and Descriptions
Relative Density
Recency
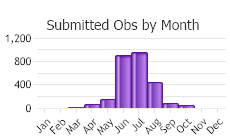
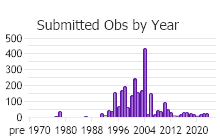
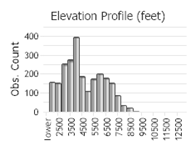 (Observations spanning multiple months or years are excluded from time charts)
(Observations spanning multiple months or years are excluded from time charts)
Migration
Although individual animals tend to use the same migration routes, no preference in habitat, relative soil moisture or vegetation is evident for the species’ movements to and from breeding pools (Beneski et al. 1986).
Habitat
Adults are found in a wide variety of habitats including semi-arid sagebrush, alpine meadows, dry woodlands, humid forests, rocky shores of mountain lakes, and disturbed agricultural areas (Nussbaum et al. 1983). Outside of the breeding season adults are primarily subterranean and have been documented to commonly move at least 600 m (1,969 ft) from the nearest breeding site on the University of Montana’s Lubrecht Experimental Forest (Maxell et al. 2009). During the breeding season adults may be found in shallow waters or under logs, rocks, and other debris near water.
National Vegetation Classification System Groups Associated with this Species
Forest and Woodland
Deciduous Forest and Woodland
Low Elevation - Xeric Forest and Woodland
Montane - Subalpine Forest and Woodland
Shrubland
Foothills - Montane Shrubland
Sagebrush Shrubland
Grassland
Lowland - Prairie Grassland
Montane - Subalpine Grassland
Wetland and Riparian
Alkaline - Saline Wetlands
Alpine Riparian and Wetland
Peatland
Riparian and Wetland Forest
Riparian Shrubland
Wet Meadow and Marsh
Recently Disturbed or Modified
Harvested Forest
Insect-Killed Forest
Recently Burned
Human Land Use
Agriculture
Developed
Food Habits
Larvae and adults feed on a variety of aquatic and terrestrial invertebrates and larvae feed on other amphibian larvae including conspecifics (Farner 1947, Anderson 1968b, Walls et al. 1993, Maxell et al. 2009). Franz (1997) identified that larvae diets consisted of ostracods/cyclops; also red water mites, insect egg masses, algae. Farner (1947) determined adult diets contained terrestrial arthropods (mostly formicid coleop, diptera) 74% and aquatic insect larvae (mostly trichop) 37%.
Reproductive Characteristics
Adults go to the breeding ponds immediately after snowmelt. In the Pacific Northwest and western Montana, it is the earliest amphibian to breed (Nussbaum et al. 1983). Breeding takes place in temporary or permanent ponds or in quiet water at the edge of lakes and streams, usually those without fish present (Funk and Dunlap 1999, Monello and Wright 1999). Like all salamanders, they have internal fertilization. Following breeding, they move to adjacent uplands.
Eggs are attached to vegetation or loose on the bottom at depths up to 0.8 m (31.5 in) and hatch in 3 to 6 weeks. Paedogenesis is unknown in this species. Larvae usually transform at the end of their first summer at low elevations or at the end of their second, third or fourth summer at high elevations and in cold waters at lower elevations (Howard and Wallace 1985, Maxell et al. 2009).
In Idaho, they have been documented breeding in February to May below 2,100 m (6,890 ft) and June to July above 2,100 m (6,890 ft). Clutch sizes are typically around 167 at lower elevations and 90 at high elevations. Metamorphose below 2,100 m (6,890 ft) occurs when SVL is 35-40 mm (1.4-1.6 in) in year 1 and above 2,100 m (6,890 ft), during year 2 to 4 when SVL 47 mm (1.9 in) (Howard and Wallace 1985). Metamorphs are typically seen in August to September (Brunson and Demaree 1951, Franz 1971). Salamanders become sexually mature when SVL is 50 mm (2.0 in).
Management
The following is taken from the Status and Conservation section for the Long-toed Salamander account in
Maxell et al. 2009Long-toed Salamanders are the most widely distributed and common amphibian species west of the Continental Divide with larvae being found in most fishless standing waterbodies with adjacent soils that provide suitable terrestrial habitat (i.e., sites that are not surrounded by extensive areas of bare rock). Their status in the front ranges east of the Continental Divide is uncertain. Risk factors relevant to the viability of populations of this species are likely to include all the general risk factors described above with the exception of harvest and commerce. Individual studies that specifically identify risk factors or other issues relevant to the conservation of Long-toed Salamanders include the following. (1) A number of studies have found adverse impacts of introduced fish on Long-toed Salamanders. Funk and Dunlap (1999) found that trout effectively excluded salamander populations from lakes in the Bitterroot Mountains. However, when fish went extinct in lakes that did not have spawning habitat salamanders were able to recolonize some of them over a twenty-year time period. In the Palouse region of northern Idaho, Monello and Wright (1999) found the presence of Long-toed Salamanders to be highly negatively correlated with the presence of a variety of fish species, including Largemouth Bass (
Micropterus salmoides), Bluegill (
Lepomis macrochirus), Channel Catfish (
Ictalurus punctatus), and Goldfish. Tyler et al. (1998a) found a similar pattern in North Cascades National Park and found that nitrogen levels were positively correlated with salamander densities in fishless lakes, apparently an indication of bottom up limitations on the food web. Similarly, Long-toed Salamanders in the central and northern portion of the Sierra Nevada Mountains are largely restricted to fishless lakes (Bradford and Gordon 1992 as cited in Knapp 1996). Tyler et al. (1998b) found that when Rainbow Trout (
Oncorhynchus mykiss) were stocked in experimental ponds with Long-toed Salamanders, larval survivorship was lower and larval body lengths were smaller than in control ponds without fish, supporting the theory that introduced trout not only impact salamanders through direct predation, but also indirectly by increasing refuge use and, thereby, reducing foraging time. (2) In a study of the Long-toed Salamander in Douglas-fir (
Pseudotsuga menziesii) forests in the Swan River Valley, McGraw (1997) found that areas where overstory removal (250-300 trees harvested per hectare) and new forestry (leave 13-25 dominant tree species per hectare and retain all snags and hardwoods) harvest techniques were applied had less ground cover, higher soil temperatures, and 75% fewer terrestrial salamanders than control plots. Interestingly, he also found that larvae were more abundant in ponds where a fraction of the pond margin was harvested than either ponds whose margins were completely harvested or ponds whose forest margins were completely intact. (3) Tallmon et al. (2000) found that gene flow among populations in the Bitterroot Mountains is greater between populations on the same mountain ridge than between populations on adjacent mountain ridges, indicating that drainages between ridges act as more of a barrier to dispersal than the steep terrain on the ridge itself. The dominance of terrestrial dispersal is also supported by the genetic analyses of Howard and Wallace (1981). (4) Fukumoto and Herrero (1998) documented mortality of a minimum of 1-2% of the adult breeding population as they crossed a roadway to the breeding site in Waterton Lakes National Park in Alberta. However, the authors suggest that actual mortality may have been considerably higher, contributing to the unusual 3:1 female biased sex ratio observed at the breeding site. (5) Blaustein et al. (1994d) found that the species has low levels of photolyase, an enzyme that repairs UV-B radiation damage to DNA. Blaustein et al. (1997) subsequently found that only 14.5% of embryos exposed to 94% of ambient UV-B radiation survived to hatching as compared to 95% survival for larvae exposed to only 10% of ambient UV-B radiation. Together these findings suggest that enhanced UV-B radiation from thinning of the ozone layer may be impacting salamander populations now or will impact them at some time in the future. (6) Sessions and Ruth (1990) found that cysts of a trematode parasite apparently caused limb deformities at the site of the cyst. This parasite has now been found in larvae collected in Montana (Pieter Johnson, Claremont Mckenna College, personal communication). (7) The extent of the use of larval Long-toed Salamanders as fishing bait is unknown in Montana, however the species is known to be used in large numbers in other states (Collins 1981) so this practice does have the potential to impact populations in Montana. Accidental introduction of larvae being used for bait may result in hybridization and genetic introgression, possibly leading to the elimination of distinct genetic makeups (Collins 1981). (8) Bradford et al. (1994) found that the LC50 pH for Pacific treefrog embryos and hatchlings exposed for 7 days averaged 4.3 and that pH levels greater than or equal to 5.0 had no significant lethal or sublethal effects.
Stewardship Responsibility
References
- Literature Cited AboveLegend:
 View Online Publication
View Online Publication Anderson, J.D. 1968b. A comparison of the food habits of Ambystoma macrodactylum sigillatum, Ambystoma macrodactylum croceum and Ambystoma tigrinum californiense. Herpetologica 24(4): 273-284.
Anderson, J.D. 1968b. A comparison of the food habits of Ambystoma macrodactylum sigillatum, Ambystoma macrodactylum croceum and Ambystoma tigrinum californiense. Herpetologica 24(4): 273-284. Beneski, J.T. Jr., E.J. Zalisko and J.H. Larsen Jr. 1986. Demography and migratory patterns of the eastern long-toed salamander, Ambystoma macrodactylum columbianum. Copeia 1986(2): 398-408.
Beneski, J.T. Jr., E.J. Zalisko and J.H. Larsen Jr. 1986. Demography and migratory patterns of the eastern long-toed salamander, Ambystoma macrodactylum columbianum. Copeia 1986(2): 398-408. Blaustein, A.R., J.M. Keisecker, D.P. Chivers, and R.G. Anthony. 1997. Ambient UV-B radiation causes deformities in amphibian embryos. Proceedings of the National Academy of Science USA 94: 13735-13737.
Blaustein, A.R., J.M. Keisecker, D.P. Chivers, and R.G. Anthony. 1997. Ambient UV-B radiation causes deformities in amphibian embryos. Proceedings of the National Academy of Science USA 94: 13735-13737. Blaustein, A.R., P.D. Hoffman, D.G. Hokit, J.M. Kiesecker, S.C. Walls, and J.B. Hays. 1994d. UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines? Proceedings of the National Academy of Sciences 91: 1791-1795.
Blaustein, A.R., P.D. Hoffman, D.G. Hokit, J.M. Kiesecker, S.C. Walls, and J.B. Hays. 1994d. UV repair and resistance to solar UV-B in amphibian eggs: a link to population declines? Proceedings of the National Academy of Sciences 91: 1791-1795. Bradford, D.F., and M.S. Gordon. 1992. Aquatic amphibians in the Sierra Nevada: current status and potential effects of acidic deposition on populations. Final Report, Contract No. A932-139. California Air Resources Board. Sacramento, CA.
Bradford, D.F., and M.S. Gordon. 1992. Aquatic amphibians in the Sierra Nevada: current status and potential effects of acidic deposition on populations. Final Report, Contract No. A932-139. California Air Resources Board. Sacramento, CA. Bradford, D.F., C. Swanson, and M.S. Gordon. 1994. Effects of low pH and aluminum on amphibians at high elevation in Sierra Nevada, California. Canadian Journal of Zoology 72: 1272-1279.
Bradford, D.F., C. Swanson, and M.S. Gordon. 1994. Effects of low pH and aluminum on amphibians at high elevation in Sierra Nevada, California. Canadian Journal of Zoology 72: 1272-1279. Brunson, R.B. and H.A. Demaree, Jr. 1951. The herpetology of the Mission Mountains, Montana. Copeia (4):306-308.
Brunson, R.B. and H.A. Demaree, Jr. 1951. The herpetology of the Mission Mountains, Montana. Copeia (4):306-308. Collins, J.P. 1981. Distribution, habitats and life history variation in the tiger salamander (Ambystoma tigrinum) in east-central and southeast Arizona. Copeia 1981(3): 666-675.
Collins, J.P. 1981. Distribution, habitats and life history variation in the tiger salamander (Ambystoma tigrinum) in east-central and southeast Arizona. Copeia 1981(3): 666-675. Farner, D.S. 1947. Notes on the food habits of the salamanders of Crater Lake, Oregon. Copeia 1947(4): 259-261.
Farner, D.S. 1947. Notes on the food habits of the salamanders of Crater Lake, Oregon. Copeia 1947(4): 259-261. Ferguson, D.E. 1961. The geographic variation of Ambystoma macrodactylum Baird, with the description of two new subspecies. American Midland Naturalist 65: 311-338.
Ferguson, D.E. 1961. The geographic variation of Ambystoma macrodactylum Baird, with the description of two new subspecies. American Midland Naturalist 65: 311-338. Franz, R. 1971. Notes on the distribution and ecology of the herpetofauna of northwestern Montana. Bulletin of the Maryland Herpetological Society 7: 1-10.
Franz, R. 1971. Notes on the distribution and ecology of the herpetofauna of northwestern Montana. Bulletin of the Maryland Herpetological Society 7: 1-10. Fukumoto, J.M. and S. Herrero. 1998. Observations of the long-toed salamander, Ambystoma macrodactylum, in Waterton Lakes National Park, Alberta. TCanadian Field Naturalist 112(4): 579-585.
Fukumoto, J.M. and S. Herrero. 1998. Observations of the long-toed salamander, Ambystoma macrodactylum, in Waterton Lakes National Park, Alberta. TCanadian Field Naturalist 112(4): 579-585. Funk, W.C. and W.W. Dunlap. 1999. Colonization of high-elevation lakes by long-toed salamanders (Ambystoma macrodactylum) after the extinction of introduced trout populations. Canadian Journal of Zoology 77: 1759-1767.
Funk, W.C. and W.W. Dunlap. 1999. Colonization of high-elevation lakes by long-toed salamanders (Ambystoma macrodactylum) after the extinction of introduced trout populations. Canadian Journal of Zoology 77: 1759-1767. Howard, J.H. and R.L. Wallace. 1981. Microgeographic variation of electrophoretic loci in populations of Ambystoma macrodactylum columbianum (Caudata: Ambystomatidae). Copeia 1981(2): 466-471.
Howard, J.H. and R.L. Wallace. 1981. Microgeographic variation of electrophoretic loci in populations of Ambystoma macrodactylum columbianum (Caudata: Ambystomatidae). Copeia 1981(2): 466-471. Howard, J.H. and R.L. Wallace. 1985. Life history characteristics of populations of the long-toed salamander (Ambystoma macrodactylum) from different altitudes. American Midland Naturalist 113(2): 361-373.
Howard, J.H. and R.L. Wallace. 1985. Life history characteristics of populations of the long-toed salamander (Ambystoma macrodactylum) from different altitudes. American Midland Naturalist 113(2): 361-373. Knapp, R.A. 1996. Non-native trout in natural lakes of the Sierra Nevada: an analysis of their distribution and impacts on native aquatic biota. In: Sierra Nevada ecosystem project: final report to Congress, volume III, assessments, commissioned reports, and background information. Davis, CA: University of California Centers for Water and Wildland Resources. Wildland Resources Center Report 38. p 363-407.
Knapp, R.A. 1996. Non-native trout in natural lakes of the Sierra Nevada: an analysis of their distribution and impacts on native aquatic biota. In: Sierra Nevada ecosystem project: final report to Congress, volume III, assessments, commissioned reports, and background information. Davis, CA: University of California Centers for Water and Wildland Resources. Wildland Resources Center Report 38. p 363-407. Maxell, B.A., P. Hendricks, M.T. Gates, and S. Lenard. 2009. Montana amphibian and reptile status assessment, literature review, and conservation plan, June 2009. Montana Natural Heritage Program. Helena, MT. 643 p.
Maxell, B.A., P. Hendricks, M.T. Gates, and S. Lenard. 2009. Montana amphibian and reptile status assessment, literature review, and conservation plan, June 2009. Montana Natural Heritage Program. Helena, MT. 643 p. McGraw, R.L., II. 1997. Timber harvest effects on metamorphosed and larval Long-toed Salamanders (Ambystoma macrodactylum). M.S. Thesis. University of Montana, Missoula, MT. 74 p.
McGraw, R.L., II. 1997. Timber harvest effects on metamorphosed and larval Long-toed Salamanders (Ambystoma macrodactylum). M.S. Thesis. University of Montana, Missoula, MT. 74 p. Nussbaum, R.A., E.D. Brodie, Jr. and R.M. Storm. 1983. Amphibians and reptiles of the Pacific Northwest. University of Idaho Press. Moscow, ID. 332 pp.
Nussbaum, R.A., E.D. Brodie, Jr. and R.M. Storm. 1983. Amphibians and reptiles of the Pacific Northwest. University of Idaho Press. Moscow, ID. 332 pp. Petranka, J.W. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington D.C. 587 pp.
Petranka, J.W. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington D.C. 587 pp. Russell, A. P. and A. M. Bauer. 2000. The Amphibians and Reptiles of Alberta: A field guide and primer of boreal herpetology. University of Calgary Press, Toronto, Ontario. 279 p.
Russell, A. P. and A. M. Bauer. 2000. The Amphibians and Reptiles of Alberta: A field guide and primer of boreal herpetology. University of Calgary Press, Toronto, Ontario. 279 p. Russell, A.P., G.L. Powell, and D.R. Hall. 1996. Growth and age of Alberta long-toed salamanders (Ambystoma macrodactylum krausei): a comparison of two methods of estimation. Canadian Journal of Zoology 74: 397-412.
Russell, A.P., G.L. Powell, and D.R. Hall. 1996. Growth and age of Alberta long-toed salamanders (Ambystoma macrodactylum krausei): a comparison of two methods of estimation. Canadian Journal of Zoology 74: 397-412. Sessions, S.K. and S.B. Ruth. 1990. Explanation for naturally occurring supernumerary limbs in amphibians. Journal of Experimental Zoology 254(1): 38-47.
Sessions, S.K. and S.B. Ruth. 1990. Explanation for naturally occurring supernumerary limbs in amphibians. Journal of Experimental Zoology 254(1): 38-47. Slater, J.R. 1936. Notes on Ambystoma gracile Baird and Ambystoma macrodactylum Baird. Copeia 1936(4): 234-236.
Slater, J.R. 1936. Notes on Ambystoma gracile Baird and Ambystoma macrodactylum Baird. Copeia 1936(4): 234-236. Tallmon, D.T., W.C. Funk, W.W. Dunlap, and F.W. Allendorf. 2000. Genetic differentiation of long-toed salamanders (Ambystoma macrodactylum) populations. Copeia 2000(1): 27-35.
Tallmon, D.T., W.C. Funk, W.W. Dunlap, and F.W. Allendorf. 2000. Genetic differentiation of long-toed salamanders (Ambystoma macrodactylum) populations. Copeia 2000(1): 27-35. Tyler, T., W.J. Liss, L.M. Ganio, G.L. Larson, R. Hoffman, E. Deimling, and G. Lomnicky. 1998a. Interaction between introduced trout and larval salamanders (Ambystoma macrodactylum) in high elevation lakes. Conservation Biology 12: 94-105.
Tyler, T., W.J. Liss, L.M. Ganio, G.L. Larson, R. Hoffman, E. Deimling, and G. Lomnicky. 1998a. Interaction between introduced trout and larval salamanders (Ambystoma macrodactylum) in high elevation lakes. Conservation Biology 12: 94-105. Tyler, T.J., W.J. Liss, R.L. Hoffman, and L.M. Ganio. 1998b. Experimental analysis of trout effects on survival, growth, and habitat use of two species of Ambystomid salamanders. Journal of Herpetology 32(3): 345-349.
Tyler, T.J., W.J. Liss, R.L. Hoffman, and L.M. Ganio. 1998b. Experimental analysis of trout effects on survival, growth, and habitat use of two species of Ambystomid salamanders. Journal of Herpetology 32(3): 345-349. Walls, S.C., J.J. Beatty, B.N. Tissot, D.G. Hokit, and A.R. Blaustein. 1993. Morphological variation and cannibalism in a larval salamander (Ambystoma macrodactylum columbianum). Canadian Journal of Zoology 71: 1543-1551.
Walls, S.C., J.J. Beatty, B.N. Tissot, D.G. Hokit, and A.R. Blaustein. 1993. Morphological variation and cannibalism in a larval salamander (Ambystoma macrodactylum columbianum). Canadian Journal of Zoology 71: 1543-1551. Werner, J.K., B.A. Maxell, P. Hendricks and D.L. Flath. 2004. Amphibians and Reptiles of Montana. Mountain Press Publishing Company: Missoula, MT. 262 pp.
Werner, J.K., B.A. Maxell, P. Hendricks and D.L. Flath. 2004. Amphibians and Reptiles of Montana. Mountain Press Publishing Company: Missoula, MT. 262 pp.
- Additional ReferencesLegend:
 View Online Publication
View Online Publication
Do you know of a citation we're missing? [WWPC] Washington Water Power Company. 1995. 1994 wildlife report Noxon Rapids and Cabinet Gorge Reservoirs. Washington Water Power Company. Spokane, WA.
[WWPC] Washington Water Power Company. 1995. 1994 wildlife report Noxon Rapids and Cabinet Gorge Reservoirs. Washington Water Power Company. Spokane, WA. Adams, M.J., B.R. Hossack, and R.A. Knapp. 2002. Distribution patterns of lentic-breeding amphibians in relation to ultraviolet radiation in western North America. Northwestern Naturalist 83:62.
Adams, M.J., B.R. Hossack, and R.A. Knapp. 2002. Distribution patterns of lentic-breeding amphibians in relation to ultraviolet radiation in western North America. Northwestern Naturalist 83:62. Adams, M.J., B.R. Hossack, and R.A. Knapp. 2005. Distribution patterns of lentic-breeding amphibians in relation to ultraviolet radiation in western North America. Ecosystems 8(5):488-500.
Adams, M.J., B.R. Hossack, and R.A. Knapp. 2005. Distribution patterns of lentic-breeding amphibians in relation to ultraviolet radiation in western North America. Ecosystems 8(5):488-500. Anderson, A.R. and J.W. Petranka. 2003. Odonate predator does not affect hatching time or morphology of embryos of two amphibians. Journal of Herpetology 37(1):65-71.
Anderson, A.R. and J.W. Petranka. 2003. Odonate predator does not affect hatching time or morphology of embryos of two amphibians. Journal of Herpetology 37(1):65-71. Anderson, J.D. 1961. The courtship behavior of Ambystoma macrodactylum croceum. Copeia 1961(2): 132-139.
Anderson, J.D. 1961. The courtship behavior of Ambystoma macrodactylum croceum. Copeia 1961(2): 132-139. Anderson, J.D. 1963. Reactions of the western mole to skin secretions of Ambystoma macrodactylum croceum. Herpetologica 19(4): 282-284.
Anderson, J.D. 1963. Reactions of the western mole to skin secretions of Ambystoma macrodactylum croceum. Herpetologica 19(4): 282-284. Anderson, J.D. 1967. A comparison of life histories of coastal and montane populations of Ambystoma macrodactylum in California. American Midland Naturalist 77(2): 323-355.
Anderson, J.D. 1967. A comparison of life histories of coastal and montane populations of Ambystoma macrodactylum in California. American Midland Naturalist 77(2): 323-355. Anderson, J.D. 1968a. Thermal histories of two populations of Ambystoma macrodactylum. Herpetologica 24(1): 29-35.
Anderson, J.D. 1968a. Thermal histories of two populations of Ambystoma macrodactylum. Herpetologica 24(1): 29-35. Anderson, J.D. 1972. Behavior of three subspecies of Ambystoma macrodactylum in a soil moisture gradient. Journal of Herpetology 6(3-4): 191-194.
Anderson, J.D. 1972. Behavior of three subspecies of Ambystoma macrodactylum in a soil moisture gradient. Journal of Herpetology 6(3-4): 191-194. Anderson, J.D. 1972. Phototactic behavior of larvae and adults of two subspecies of Ambystoma macrodactylum. Herpetologica 28(3):222-226.
Anderson, J.D. 1972. Phototactic behavior of larvae and adults of two subspecies of Ambystoma macrodactylum. Herpetologica 28(3):222-226. Anderson, J.D. and R.E. Graham. 1967. Vertical migration and stratification of larval Ambystoma. Copeia 2:371-374.
Anderson, J.D. and R.E. Graham. 1967. Vertical migration and stratification of larval Ambystoma. Copeia 2:371-374. Anderson, M.E. 1977. Aspects of the ecology of two sympatric species of Thamnophis and heavy metal accumulation with the species. M.S. thesis, University of Montana, Missoula. 147 pp.
Anderson, M.E. 1977. Aspects of the ecology of two sympatric species of Thamnophis and heavy metal accumulation with the species. M.S. thesis, University of Montana, Missoula. 147 pp. Bailey, J.L. 1948. Supplementary observations on the geographic variation of Ambystoma macrodactylum. Herpetologica 4: 171-174.
Bailey, J.L. 1948. Supplementary observations on the geographic variation of Ambystoma macrodactylum. Herpetologica 4: 171-174. Belden, L.K., E.L. Wildy, and A.R. Balustein. 2000a. Growth, survival and behaviour of larval long-toed salamanders (Ambystoma macrodactylum) exposed to ambient levels of UV-B radiation. Journal of Zoology : Proceedings of the Zoological 251(4): 473.
Belden, L.K., E.L. Wildy, and A.R. Balustein. 2000a. Growth, survival and behaviour of larval long-toed salamanders (Ambystoma macrodactylum) exposed to ambient levels of UV-B radiation. Journal of Zoology : Proceedings of the Zoological 251(4): 473. Beneski, J.T., Jr., J.H. Larsen Jr., and B.T. Miller. 1995. Variation in the feeding kinematics of mole salamanders (Ambystomatidae: Ambystoma). Canadian Journal of Zoology 73(2): 353-366.
Beneski, J.T., Jr., J.H. Larsen Jr., and B.T. Miller. 1995. Variation in the feeding kinematics of mole salamanders (Ambystomatidae: Ambystoma). Canadian Journal of Zoology 73(2): 353-366. Berner, N.J. and R.L. Ingermann. 1988. Interaction of exogenous proteins with the jelly coat of eggs from the salamander, Ambystoma macrodactylum. Comparative Biochemistry and Physiology 90A(2): 265-268.
Berner, N.J. and R.L. Ingermann. 1988. Interaction of exogenous proteins with the jelly coat of eggs from the salamander, Ambystoma macrodactylum. Comparative Biochemistry and Physiology 90A(2): 265-268. Berner, N.J. and R.L. Ingermann. 1990. Role of sialic acid in exogenous protein accumulation and water retention by the egg jelly of the salamander Ambystoma macrodactylum. Journal of Experimental Zoology 256(1): 38-43.
Berner, N.J. and R.L. Ingermann. 1990. Role of sialic acid in exogenous protein accumulation and water retention by the egg jelly of the salamander Ambystoma macrodactylum. Journal of Experimental Zoology 256(1): 38-43. Blackwell, E.A., R.A. Angus, G.R. Cline, and K.R. Marion. 2003. Natural growth rates of Ambystoma maculatum in Alabama. Journal of Herpetology 37(3):608-612.
Blackwell, E.A., R.A. Angus, G.R. Cline, and K.R. Marion. 2003. Natural growth rates of Ambystoma maculatum in Alabama. Journal of Herpetology 37(3):608-612. Blaustein, A.R., E.L. Wildy, L.K. Beldan, and A. Hatch. 2001. Influence of abiotic and biotic factors on amphibians in ephemeral ponds with special reference to long-toed salamanders (Ambystoma macrodactylum). Israel Journal of Zoology 47(4):333-345.
Blaustein, A.R., E.L. Wildy, L.K. Beldan, and A. Hatch. 2001. Influence of abiotic and biotic factors on amphibians in ephemeral ponds with special reference to long-toed salamanders (Ambystoma macrodactylum). Israel Journal of Zoology 47(4):333-345. Blaustein, A.R., J.J. Beatty, H. Deanna, and R.M. Storm. 1995. The biology of amphibians and reptiles in old-growth forests in the Pacific Northwest. General Technical Report PNW-GTR-337. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 98 p.
Blaustein, A.R., J.J. Beatty, H. Deanna, and R.M. Storm. 1995. The biology of amphibians and reptiles in old-growth forests in the Pacific Northwest. General Technical Report PNW-GTR-337. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 98 p. Boone, M.D., E.E. Little, and R.D. Semlitsch. 2004. Overwintered bullfrog tadpoles negatively affect salamanders and anurans in native amphibian communities. Copeia 3:683-690.
Boone, M.D., E.E. Little, and R.D. Semlitsch. 2004. Overwintered bullfrog tadpoles negatively affect salamanders and anurans in native amphibian communities. Copeia 3:683-690. Boundy, J. 2001. Herpetofaunal surveys in the Clark Fork Valley region, Montana. Herpetological Natural History 8: 15-26.
Boundy, J. 2001. Herpetofaunal surveys in the Clark Fork Valley region, Montana. Herpetological Natural History 8: 15-26. Branch, L.C. and R. Altig. 1981. Nocturnal stratification of three species of Ambystoma larvae. Copeia 1981(4): 870-873.
Branch, L.C. and R. Altig. 1981. Nocturnal stratification of three species of Ambystoma larvae. Copeia 1981(4): 870-873. Brodie, E.D., Jr. 1977. Salamander antipredator postures. Copeia 1977(3): 523-535.
Brodie, E.D., Jr. 1977. Salamander antipredator postures. Copeia 1977(3): 523-535. Brodman, R. 1993. The efffect of acidity on interactions of Ambystoma salamander larvae. Journal of Freshwater Ecology 8(3): 209-214.
Brodman, R. 1993. The efffect of acidity on interactions of Ambystoma salamander larvae. Journal of Freshwater Ecology 8(3): 209-214. Brodman, R. 2004. Intraguild predation on congeners affects size, aggression, and survival among Ambystoma salamander larvae. Journal of Herpetology 38(1):21-26.
Brodman, R. 2004. Intraguild predation on congeners affects size, aggression, and survival among Ambystoma salamander larvae. Journal of Herpetology 38(1):21-26. Brodman, R., J. Ogger, M. Kolaczyk, R.A. Pulver, A.J. Long, T. Bogard. 2003. Mosquito control by pond-breeding salamander larvae. Herpetologocal Review 34(2):116-119.
Brodman, R., J. Ogger, M. Kolaczyk, R.A. Pulver, A.J. Long, T. Bogard. 2003. Mosquito control by pond-breeding salamander larvae. Herpetologocal Review 34(2):116-119. Brunson, R.B. 1955. Check list of the amphibians and reptiles of Montana. Proceedings of the Montana Academy of Sciences 15: 27-29.
Brunson, R.B. 1955. Check list of the amphibians and reptiles of Montana. Proceedings of the Montana Academy of Sciences 15: 27-29. Bull, E.L., J.W. Deal, and J.E. Hohmann. 2001. Avian and amphibian use of fenced and unfenced stock ponds in northeastern Oregon forests. US Forest Service Research Paper PNW 539: 1-9.
Bull, E.L., J.W. Deal, and J.E. Hohmann. 2001. Avian and amphibian use of fenced and unfenced stock ponds in northeastern Oregon forests. US Forest Service Research Paper PNW 539: 1-9. Calfee, R.D., C.M. Bridges, and E.E. Little. 2006. Sensitivity of two salamaners (Ambystoma) species to ultraviolet radiation. Journal of Herpetology 40(1):35-42.
Calfee, R.D., C.M. Bridges, and E.E. Little. 2006. Sensitivity of two salamaners (Ambystoma) species to ultraviolet radiation. Journal of Herpetology 40(1):35-42. Carl, G.C. 1942. The long-toed salamander on Vancouver Island. Copeia 1942(1): 56.
Carl, G.C. 1942. The long-toed salamander on Vancouver Island. Copeia 1942(1): 56. Carl, G.C. and I.M. Cowan. 1945. Notes on the salamanders of British Columbia. Copeia 1945(1): 43-44.
Carl, G.C. and I.M. Cowan. 1945. Notes on the salamanders of British Columbia. Copeia 1945(1): 43-44. Chivers, D.P., E.L. Wildy, and A.R. Blaustein. 1997. Eastern long-toed salamander (Ambystoma macrodactylum columbianum) larvae recognize cannabilistic conspecifics. Ethology 103: 187-197.
Chivers, D.P., E.L. Wildy, and A.R. Blaustein. 1997. Eastern long-toed salamander (Ambystoma macrodactylum columbianum) larvae recognize cannabilistic conspecifics. Ethology 103: 187-197. Chivers, D.P., J.M. Kiesecker, M.T. Anderson, E.L. Wildy, and A.R. Blaustein. 1996. Avoidance response of a terrestrial salamander (Ambystoma macrodactylum) to chemical alarm cues. Journal of Chemical Ecology 22(9): 1709-1716.
Chivers, D.P., J.M. Kiesecker, M.T. Anderson, E.L. Wildy, and A.R. Blaustein. 1996. Avoidance response of a terrestrial salamander (Ambystoma macrodactylum) to chemical alarm cues. Journal of Chemical Ecology 22(9): 1709-1716. Clark, K.L. 1985. Responses of spotted salamander (Ambystoma maculatum) populations in central Ontario (Candada) to habitat acidity. Canadian Field Naturalist 100(4): 463-469.
Clark, K.L. 1985. Responses of spotted salamander (Ambystoma maculatum) populations in central Ontario (Candada) to habitat acidity. Canadian Field Naturalist 100(4): 463-469. Cook, R. and K. Boland. 2005. A comparison of approaches to counting spotted salamander (Ambystoma maculatum) egg masses in vernal ponds. Herpetological Review 36(3):272-274.
Cook, R. and K. Boland. 2005. A comparison of approaches to counting spotted salamander (Ambystoma maculatum) egg masses in vernal ponds. Herpetological Review 36(3):272-274. Cope, E.D. 1867. A review of the species of the Amblystomidae. Proceedings of the Academy of Natural Sciences of Philadelphia 19: 166-211.
Cope, E.D. 1867. A review of the species of the Amblystomidae. Proceedings of the Academy of Natural Sciences of Philadelphia 19: 166-211. Cope, E.D. 1875. Check-list of North American Batrachia and Reptilia; with a systematic list of the higher groups, and an essay on geographical distribution. Based on the specimens contained in the U.S. National Museum. U.S. Natioanl Museum Bulletin 1: 1-104.
Cope, E.D. 1875. Check-list of North American Batrachia and Reptilia; with a systematic list of the higher groups, and an essay on geographical distribution. Based on the specimens contained in the U.S. National Museum. U.S. Natioanl Museum Bulletin 1: 1-104. Cope, E.D. 1889. The Batrachia of North America. Bulletin of the U.S. National Museum 34: 1-525, figs. 1-119, pls. 1-86.
Cope, E.D. 1889. The Batrachia of North America. Bulletin of the U.S. National Museum 34: 1-525, figs. 1-119, pls. 1-86. Corn, P.S., B.R. Hossack, E. Muths, D.A. Palta, C.R. Peterson, and A.L. Gallant. 2005. Status of amphibians on the Continental Divide: aurveys on a transect from Montana to Colorado, USA. Alytes 22(3-4):85-94.
Corn, P.S., B.R. Hossack, E. Muths, D.A. Palta, C.R. Peterson, and A.L. Gallant. 2005. Status of amphibians on the Continental Divide: aurveys on a transect from Montana to Colorado, USA. Alytes 22(3-4):85-94. Crother, B.I. (ed.) 2008. Scientific and standard English names of amphibians and reptiles of North America north of Mexico. SSAR Herpetological Circular No. 37:1-84.
Crother, B.I. (ed.) 2008. Scientific and standard English names of amphibians and reptiles of North America north of Mexico. SSAR Herpetological Circular No. 37:1-84. Cunnington, D.C. and R.J. Brooks. 2000. Optimal egg size theory: Does predation by fish affect egg size in Ambytroma maculatum? Journal of Herpetology 34(1):46-53.
Cunnington, D.C. and R.J. Brooks. 2000. Optimal egg size theory: Does predation by fish affect egg size in Ambytroma maculatum? Journal of Herpetology 34(1):46-53. Dempster, W.T. 1930. The growth of larvae of Ambystoma maculatum under natural conditions. Biology Bulletin 58: 182-192.
Dempster, W.T. 1930. The growth of larvae of Ambystoma maculatum under natural conditions. Biology Bulletin 58: 182-192. Dougherty, C.K., D.A. Vaala, and G.R. Smith. 2005. Within-pond oviposition site selection in two spring-breeding amphibians (Ambystoma maculatum and Rana sylvatica). Journal of Freshwater Ecology 20(4):781-782.
Dougherty, C.K., D.A. Vaala, and G.R. Smith. 2005. Within-pond oviposition site selection in two spring-breeding amphibians (Ambystoma maculatum and Rana sylvatica). Journal of Freshwater Ecology 20(4):781-782. Ducey, P.K. 1989. Agonistic behavior and biting during intraspecific encounters in Ambystoma salamanders. Herpetologica 45(2): 155-160.
Ducey, P.K. 1989. Agonistic behavior and biting during intraspecific encounters in Ambystoma salamanders. Herpetologica 45(2): 155-160. Dunlap, W.W. and F.W. Allendorf. No Date. Population structure of the long-toed salamander. Unpublished report. University of Montana. Missoula, MT. 24 p. + 8 figs.
Dunlap, W.W. and F.W. Allendorf. No Date. Population structure of the long-toed salamander. Unpublished report. University of Montana. Missoula, MT. 24 p. + 8 figs. Efford, I.E. and J.A. Mathias. 1969. A comparison of two salamander populations in Marion Lake, British Columbia. Copeia 1969: 723-735.
Efford, I.E. and J.A. Mathias. 1969. A comparison of two salamander populations in Marion Lake, British Columbia. Copeia 1969: 723-735. Faccio, S.D. 2003. Postbreeding emigration and habitat use bu Jefferson and Spotted Salamanders in Vermont. Journal of Herpetology 37(3):479-489.
Faccio, S.D. 2003. Postbreeding emigration and habitat use bu Jefferson and Spotted Salamanders in Vermont. Journal of Herpetology 37(3):479-489. Farmer, P. and S.B. Heath. 1987. Wildlife baseline inventory, Rock Creek study area, Sanders County, Montana. Western Technology and Engineering, Inc. Helena, MT.
Farmer, P. and S.B. Heath. 1987. Wildlife baseline inventory, Rock Creek study area, Sanders County, Montana. Western Technology and Engineering, Inc. Helena, MT. Farmer, Patrick J., and Thomas W. Butts, Western Technology & Eng., Inc., Helena, MT., 1994, McDonald Project Terrestrial Wildlife Study, November 1989 - November 1993. April 1994. In McDonald Gold Project: Wildlife & Fisheries. [#18]. Seven-up Pete Joint Venture, Lincoln, MT. Unpub. No date.
Farmer, Patrick J., and Thomas W. Butts, Western Technology & Eng., Inc., Helena, MT., 1994, McDonald Project Terrestrial Wildlife Study, November 1989 - November 1993. April 1994. In McDonald Gold Project: Wildlife & Fisheries. [#18]. Seven-up Pete Joint Venture, Lincoln, MT. Unpub. No date. Ferguson, D.E. 1963. Ambystoma macrodactylum. Catalogue of American Amphibians and Reptiles 4.1-4.2.
Ferguson, D.E. 1963. Ambystoma macrodactylum. Catalogue of American Amphibians and Reptiles 4.1-4.2. Figiel, C.R. Jr. and R.D. Semlitsch. 1990. Population variation in survival and metamorphosis of larval salamanders (Ambystoma maculatum) in the presence and absence of fish predation. Copeia 1990(3): 818-826.
Figiel, C.R. Jr. and R.D. Semlitsch. 1990. Population variation in survival and metamorphosis of larval salamanders (Ambystoma maculatum) in the presence and absence of fish predation. Copeia 1990(3): 818-826. Fukumoto, J.M. 1995. Long-toed salamander (Ambystoma macrodactylum) ecology and mangement in Waterton Lakes National Park. M.S. Thesis. Faculty of Environmental Design, University of Calgary, Calgary, Alberta.
Fukumoto, J.M. 1995. Long-toed salamander (Ambystoma macrodactylum) ecology and mangement in Waterton Lakes National Park. M.S. Thesis. Faculty of Environmental Design, University of Calgary, Calgary, Alberta. Funk, W.C., D.A. Tallmon, and F.W. Allendorf. 1999. Small effective population size in the long-toed salamander. Molecular Ecology 8: 1633-1640.
Funk, W.C., D.A. Tallmon, and F.W. Allendorf. 1999. Small effective population size in the long-toed salamander. Molecular Ecology 8: 1633-1640. Galloway, B.T. 2014. Feasibility assessment for translocation of imperiled Bull Trout populations in Glacier National Park, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 110 p.
Galloway, B.T. 2014. Feasibility assessment for translocation of imperiled Bull Trout populations in Glacier National Park, Montana. M.Sc. Thesis. Bozeman, MT: Montana State University. 110 p. Gibson, J.D. and D.A. Merkle. 2005. Ambystoma maculatum (Spotted Salamander). Reproduction. Herpetological Review 36:294.
Gibson, J.D. and D.A. Merkle. 2005. Ambystoma maculatum (Spotted Salamander). Reproduction. Herpetological Review 36:294. Graham, K.L. 1997. Habitat use of long-toed salamanders (Ambystoma macrodactylum) at three different scales. M.S. Thesis, University of Guelph, Guelph, ON. 71pp.
Graham, K.L. 1997. Habitat use of long-toed salamanders (Ambystoma macrodactylum) at three different scales. M.S. Thesis, University of Guelph, Guelph, ON. 71pp. Graham, K.L. and G.L. Powell. 1999. Status of the Long-toed salamander (Ambystoma macrodactylum) in Alberta. Alberta Wildlife Status Report No. 22., Edmonton, AB. 19p.
Graham, K.L. and G.L. Powell. 1999. Status of the Long-toed salamander (Ambystoma macrodactylum) in Alberta. Alberta Wildlife Status Report No. 22., Edmonton, AB. 19p. Grant, E.H.C. and P. Nanjappa. 2006. Adressing errors in identification of Ambystoma maculatum (spotted salamanders) using spot patterns. Herpetological Review 37(1):57-60.
Grant, E.H.C. and P. Nanjappa. 2006. Adressing errors in identification of Ambystoma maculatum (spotted salamanders) using spot patterns. Herpetological Review 37(1):57-60. Greer, K.R. 1955. Yearly food habits of the River Otter in the Thompson Lakes region, northwestern Montana, as indicated by scat analysis. Am. Midl. Nat. 54(2):299-313.
Greer, K.R. 1955. Yearly food habits of the River Otter in the Thompson Lakes region, northwestern Montana, as indicated by scat analysis. Am. Midl. Nat. 54(2):299-313. Hamilton, I.M., K.L. Graham, L. Powell, and A. Russell. 1996. The range of long-toed salamanders in northwestern Alberta. Alberta Environmental Protection, Fish and Wildlife Service, Edmonton, AB. 186 pp.
Hamilton, I.M., K.L. Graham, L. Powell, and A. Russell. 1996. The range of long-toed salamanders in northwestern Alberta. Alberta Environmental Protection, Fish and Wildlife Service, Edmonton, AB. 186 pp. Hanauska-Brown, L., B.A. Maxell, A. Petersen, and S. Story. 2014. Diversity Monitoring in Montana 2008-2010 Final Report. Montana Fish, Wildlife & Parks. Helena, MT. 78 pp.
Hanauska-Brown, L., B.A. Maxell, A. Petersen, and S. Story. 2014. Diversity Monitoring in Montana 2008-2010 Final Report. Montana Fish, Wildlife & Parks. Helena, MT. 78 pp. Hendricks, P. 1997. Lee Metcalf National Wildlife Refuge preliminary amphibian and reptile investigations: 1996. Montana Natural Heritage Program, Helena, MT. 21 p.
Hendricks, P. 1997. Lee Metcalf National Wildlife Refuge preliminary amphibian and reptile investigations: 1996. Montana Natural Heritage Program, Helena, MT. 21 p. Hendricks, P. 2000. Amphibian and reptile survey of the Thompson Chain of Lakes. A report to the Montana Department of Fish, Wildlife, and Parks. Montana Natural Heritage Program, Helena, MT. 15 p.
Hendricks, P. 2000. Amphibian and reptile survey of the Thompson Chain of Lakes. A report to the Montana Department of Fish, Wildlife, and Parks. Montana Natural Heritage Program, Helena, MT. 15 p. Hendricks, P. and J.D. Reichel. 1996a. Amphibian and reptile survey of the Bitterroot National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 95 p.
Hendricks, P. and J.D. Reichel. 1996a. Amphibian and reptile survey of the Bitterroot National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 95 p. Hilliard, J., H. Minkus, and M. Weber. 1997. Amphibian survey of the Birch Creek drainage, Beaverhead County. Wildland Studies Project, San Francisco State University, San Francisco, CA. 12 p.
Hilliard, J., H. Minkus, and M. Weber. 1997. Amphibian survey of the Birch Creek drainage, Beaverhead County. Wildland Studies Project, San Francisco State University, San Francisco, CA. 12 p. Hoffman, R.L., G.L. Larson, and B.J. Brokes. 2003. Habitat segregation of Ambystoma gracile and Ambystoma macrodactylum in mountain ponds and lakes, Mt. Ranier National Park, Washington, USA. Journal of Herpetology 37(1): 24-34.
Hoffman, R.L., G.L. Larson, and B.J. Brokes. 2003. Habitat segregation of Ambystoma gracile and Ambystoma macrodactylum in mountain ponds and lakes, Mt. Ranier National Park, Washington, USA. Journal of Herpetology 37(1): 24-34. Homan, R.N., J.M. Reed, and B.S. Windmiller. 2005. Analysis of spotted salamander (Ambystroma maculatum) growth rates based on long-bone growth rings. Journal of Herpetology 37(3):617-621.
Homan, R.N., J.M. Reed, and B.S. Windmiller. 2005. Analysis of spotted salamander (Ambystroma maculatum) growth rates based on long-bone growth rings. Journal of Herpetology 37(3):617-621. Hossack, B. 2002. Ambystoma macrodactylum krausei (northern long-toed salamander) vocalization. Herpetological Review 33(2): 121.
Hossack, B. 2002. Ambystoma macrodactylum krausei (northern long-toed salamander) vocalization. Herpetological Review 33(2): 121. Hossack, B., D. Pilliod, and P.S. Corn. 2001b. Preliminary amphibian surveys of the National Bison Range, Lost Trail National Wildife Refuge, and Swan River National Wildlife Refuge: 2001. USGS Northern Rocky Mountain Science Center, Aldo Leopold Wilderness Research Institute, Missoula, MT. 15 p.
Hossack, B., D. Pilliod, and P.S. Corn. 2001b. Preliminary amphibian surveys of the National Bison Range, Lost Trail National Wildife Refuge, and Swan River National Wildlife Refuge: 2001. USGS Northern Rocky Mountain Science Center, Aldo Leopold Wilderness Research Institute, Missoula, MT. 15 p. Hossack, B.R. 2006. Amphibians and wildfire in the U.S. Northwest. International Journal of Wilderness 12(1):26.
Hossack, B.R. 2006. Amphibians and wildfire in the U.S. Northwest. International Journal of Wilderness 12(1):26. Hossack, B.R. and P.S. Corn. 2004. Responses of pond-breeding amphibians to wildfire in Glacier National Park. Abstract. Northwestern Naturalist 85:78.
Hossack, B.R. and P.S. Corn. 2004. Responses of pond-breeding amphibians to wildfire in Glacier National Park. Abstract. Northwestern Naturalist 85:78. Hossack, Blake R. 2011. Interactive effects of wildfire and disturbance history on amphibians and their parasites. PhD Dissertation. University of Montana. Missoula, MT.
Hossack, Blake R. 2011. Interactive effects of wildfire and disturbance history on amphibians and their parasites. PhD Dissertation. University of Montana. Missoula, MT. Hossack,B.R., W.R. Gould, D.A. Patla, E. Muths, R. Daley, K. Legg, and P.S. Corn. 2015. Trends in Rocky Mountain amphibians and the role of beaver as a keystone species. Biological Conservation 187:260-269.
Hossack,B.R., W.R. Gould, D.A. Patla, E. Muths, R. Daley, K. Legg, and P.S. Corn. 2015. Trends in Rocky Mountain amphibians and the role of beaver as a keystone species. Biological Conservation 187:260-269. Howard, J.H. 1979. Ecological, genetic and physiological variation in populations of the long-toed salamander (Ambystoma macrodactylum columbianum) from different altitudes. Ph.D. dissertation, University of Idaho. Moscow, Idaho. 82pp.
Howard, J.H. 1979. Ecological, genetic and physiological variation in populations of the long-toed salamander (Ambystoma macrodactylum columbianum) from different altitudes. Ph.D. dissertation, University of Idaho. Moscow, Idaho. 82pp. Howard, J.H. and J.R. Stauffer. 1983. Critical thermal maxima in populations of Ambystoma macrodactylum from different elevations. Journal of Herpetology 17(4): 402-404.
Howard, J.H. and J.R. Stauffer. 1983. Critical thermal maxima in populations of Ambystoma macrodactylum from different elevations. Journal of Herpetology 17(4): 402-404. Howard, J.H. and R.L. Wallace. 1980. Effects of altitude on selected blood parameters in populations of the salamander Ambystoma macrodactylum. Comparative Biochemistry and Physiology 65A(2): 243-245.
Howard, J.H. and R.L. Wallace. 1980. Effects of altitude on selected blood parameters in populations of the salamander Ambystoma macrodactylum. Comparative Biochemistry and Physiology 65A(2): 243-245. Husting, E.L. 1965. Survival and breeding structure in a population of Ambystoma maculatum. Copeia 1965: 352-362.
Husting, E.L. 1965. Survival and breeding structure in a population of Ambystoma maculatum. Copeia 1965: 352-362. Ingermann, R.L., D.C. Bencic, and P. Verrell. 2002. Methoxychlor alters the predator-prey relationship between dragonfly naiads and salamander larvae. Bulletin of Environmental Contamination and Toxicology 68(6):771-778.
Ingermann, R.L., D.C. Bencic, and P. Verrell. 2002. Methoxychlor alters the predator-prey relationship between dragonfly naiads and salamander larvae. Bulletin of Environmental Contamination and Toxicology 68(6):771-778. Johnson, P.T.J., K.B. Lunde, E.M. Thurman, E.G. Ritchie, S.N. Wray, D.R. Sutherland, J.M. Kapfer, T.J. Frest, J. Bowerman, and A.R. Blaustein. 2002. Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecological Monographs 72(2):151-168.
Johnson, P.T.J., K.B. Lunde, E.M. Thurman, E.G. Ritchie, S.N. Wray, D.R. Sutherland, J.M. Kapfer, T.J. Frest, J. Bowerman, and A.R. Blaustein. 2002. Parasite (Ribeiroia ondatrae) infection linked to amphibian malformations in the western United States. Ecological Monographs 72(2):151-168. Jones, Lawrence L. C., W. P. Leonard and D. H. Olson, eds. 2005. Amphibians of the Pacific Northwest. Seattle Audubon Society: Seattle, WA, 227 pp.
Jones, Lawrence L. C., W. P. Leonard and D. H. Olson, eds. 2005. Amphibians of the Pacific Northwest. Seattle Audubon Society: Seattle, WA, 227 pp. Joslin, Gayle, and Heidi B. Youmans. 1999. Effects of recreation on Rocky Mountain wildlife: a review for Montana. [Montana]: Montana Chapter of the Wildlife Society.
Joslin, Gayle, and Heidi B. Youmans. 1999. Effects of recreation on Rocky Mountain wildlife: a review for Montana. [Montana]: Montana Chapter of the Wildlife Society. Kenison, E.K. 2014. Predator-prey interactions between introduced trout and Long-Toed Salamanders and ways to mitigate noncomsumptive effects. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p.
Kenison, E.K. 2014. Predator-prey interactions between introduced trout and Long-Toed Salamanders and ways to mitigate noncomsumptive effects. M.Sc. Thesis. Bozeman, MT: Montana State University. 100 p. Kenison, E.K., A.R. Litt, D.S. Pilliod, and T.E. McMahon. 2016. Larval Long-Toed Salamanders incur nonconsumptive effects in the presence of nonnative trout. Ecosphere 7(5).
Kenison, E.K., A.R. Litt, D.S. Pilliod, and T.E. McMahon. 2016. Larval Long-Toed Salamanders incur nonconsumptive effects in the presence of nonnative trout. Ecosphere 7(5). Kezer, J. and D.S. Farner. 1955. Life history patterns of the salamander Ambystoma macrodactylum in the high Cascade Mountains of southern Oregon. Copeia 1955(2): 127-131.
Kezer, J. and D.S. Farner. 1955. Life history patterns of the salamander Ambystoma macrodactylum in the high Cascade Mountains of southern Oregon. Copeia 1955(2): 127-131. Knudsen, J.W. 1960. The courtship and egg mass of Ambystoma gracile and Ambystoma macrodactylum. Copeia 1960(1): 44-46.
Knudsen, J.W. 1960. The courtship and egg mass of Ambystoma gracile and Ambystoma macrodactylum. Copeia 1960(1): 44-46. Kraus, F. 1988. An empirical evaluation of the use of the ontogeny polarization criterion in phylogenetic inference. Systematic Zoology 37(2): 106-141.
Kraus, F. 1988. An empirical evaluation of the use of the ontogeny polarization criterion in phylogenetic inference. Systematic Zoology 37(2): 106-141. Kutka, F.J. 1994. Low pH effects of swimming activity of Ambystoma salamander larvae. Environment, Toxicology and Chemistry 13(11): 1821-1824.
Kutka, F.J. 1994. Low pH effects of swimming activity of Ambystoma salamander larvae. Environment, Toxicology and Chemistry 13(11): 1821-1824. Laselle, B.T. 2000. Association of wetland area with breeding activity for multiple amphibian species. Undergraduate Honors Thesis. Carroll College, Helena, MT. 18 p.
Laselle, B.T. 2000. Association of wetland area with breeding activity for multiple amphibian species. Undergraduate Honors Thesis. Carroll College, Helena, MT. 18 p. Leonard, W.P., and K.O. Richter. 1994. Western long-toed salamander demographics and oviposition in a small vernal wetland of the Puget Sound lowlands. Northwest Science 68(2): 135.
Leonard, W.P., and K.O. Richter. 1994. Western long-toed salamander demographics and oviposition in a small vernal wetland of the Puget Sound lowlands. Northwest Science 68(2): 135. Manville, R.H. 1957. Amphibians and reptiles of Glacier National Park, Montana. Copeia 1957: 308-309.
Manville, R.H. 1957. Amphibians and reptiles of Glacier National Park, Montana. Copeia 1957: 308-309. Marnell, L. E. 1997. Herpetofauna of Glacier National Park. Northwestern Naturalist 78:17-33.
Marnell, L. E. 1997. Herpetofauna of Glacier National Park. Northwestern Naturalist 78:17-33. Marnell, L.F. 1996. Amphibian survey of Glacier National Park, Montana. Abstract. Intermountain Journal of Sciences 2(2): 52.
Marnell, L.F. 1996. Amphibian survey of Glacier National Park, Montana. Abstract. Intermountain Journal of Sciences 2(2): 52. Maxell, B. A. 2000. Management of Montana's amphibians: a review of factors that may present a risk to population viability and accounts on the identification, distribution, taxonomy, habitat use, natural history, and the status and conservation of individual species. Report to USFS Region 1, Order Number 43-0343-0-0224. University of Montana, Wildlife Biology Program. Missoula, MT. 161 p.
Maxell, B. A. 2000. Management of Montana's amphibians: a review of factors that may present a risk to population viability and accounts on the identification, distribution, taxonomy, habitat use, natural history, and the status and conservation of individual species. Report to USFS Region 1, Order Number 43-0343-0-0224. University of Montana, Wildlife Biology Program. Missoula, MT. 161 p. Maxell, B.A. 2002a. Amphibian and aquatic reptile inventories in watersheds in the South and Middle Forks of the Flathead River drainage that contain lakes being considered for application of piscicides and subsequent stocking of west slope cutthroat trout. Report to the Region 1 Office of the U.S. Forest Service and the Montana Department of Fish, Wildlife, and Parks. Montana Cooperative Wildlife Research Unit and Wildlife Biology Program, University of Montana, Missoula, MT. 62 pp.
Maxell, B.A. 2002a. Amphibian and aquatic reptile inventories in watersheds in the South and Middle Forks of the Flathead River drainage that contain lakes being considered for application of piscicides and subsequent stocking of west slope cutthroat trout. Report to the Region 1 Office of the U.S. Forest Service and the Montana Department of Fish, Wildlife, and Parks. Montana Cooperative Wildlife Research Unit and Wildlife Biology Program, University of Montana, Missoula, MT. 62 pp. Maxell, B.A., J.K. Werner, P. Hendricks, and D.L. Flath. 2003. Herpetology in Montana: a history, status summary, checklists, dichotomous keys, accounts for native, potentially native, and exotic species, and indexed bibliography. Society for Northwestern Vertebrate Biology, Northwest Fauna Number 5. Olympia, WA. 135 p.
Maxell, B.A., J.K. Werner, P. Hendricks, and D.L. Flath. 2003. Herpetology in Montana: a history, status summary, checklists, dichotomous keys, accounts for native, potentially native, and exotic species, and indexed bibliography. Society for Northwestern Vertebrate Biology, Northwest Fauna Number 5. Olympia, WA. 135 p. McAvoy, S.A. 2003. Wetland characteristics associated with amphibian presence in the Rocky Mountain Region. Undergraduate Honors Thesis. Carroll College, Helena, MT. 23 p.
McAvoy, S.A. 2003. Wetland characteristics associated with amphibian presence in the Rocky Mountain Region. Undergraduate Honors Thesis. Carroll College, Helena, MT. 23 p. McCaffery, R., R.E. Russell, B.R. Hossack. 2021. Enigmatic near-extirpation in a boreal toad metapopulation in northwestern Montana. The Journal of Wildlife Management 85(5):953-963.
McCaffery, R., R.E. Russell, B.R. Hossack. 2021. Enigmatic near-extirpation in a boreal toad metapopulation in northwestern Montana. The Journal of Wildlife Management 85(5):953-963. McCoy, K.A., R.N. Harris. 2003. Integrating development stability analysis and current amphibian monitoring techniques: an experimental evaluation with the salamander Ambystoma maculatum. Herpetologica 59(1): 22-36.
McCoy, K.A., R.N. Harris. 2003. Integrating development stability analysis and current amphibian monitoring techniques: an experimental evaluation with the salamander Ambystoma maculatum. Herpetologica 59(1): 22-36. McGraw, R.L. 1997. Timber harvest effects on long-toed salamanders (Ambystoma macrodactylum). Abstract from SSAR meeting in Seattle, Washington.
McGraw, R.L. 1997. Timber harvest effects on long-toed salamanders (Ambystoma macrodactylum). Abstract from SSAR meeting in Seattle, Washington. McLaughlin, G.S. 1999. Draft revised recovery plan for the Santa Cruz long-toed salamander. U.S. Fish and Wildlife Service Region 1. Portland, Oregon.
McLaughlin, G.S. 1999. Draft revised recovery plan for the Santa Cruz long-toed salamander. U.S. Fish and Wildlife Service Region 1. Portland, Oregon. Menninger, K.L. 2004. The effects of hydroperiod on the head morphology of long-toed salamanders (Ambystoma macrodactylum). Undergraduate Honors Thesis. Carroll College, Helena, MT. 20 p.
Menninger, K.L. 2004. The effects of hydroperiod on the head morphology of long-toed salamanders (Ambystoma macrodactylum). Undergraduate Honors Thesis. Carroll College, Helena, MT. 20 p. Miller, J. D. 1975. Interspecific food relationships of anurans in northwestern Montana and fluoride accumulation in amphibians and reptiles in northwestern Montana. M.S. thesis. University of Montana, Missoula, MT. 105 p.
Miller, J. D. 1975. Interspecific food relationships of anurans in northwestern Montana and fluoride accumulation in amphibians and reptiles in northwestern Montana. M.S. thesis. University of Montana, Missoula, MT. 105 p. Miller, M. 1995. Amphibian survey Birch Creek July 1995. Wildland Studies Project, San Francisco State University, San Francisco, CA. 9 p.
Miller, M. 1995. Amphibian survey Birch Creek July 1995. Wildland Studies Project, San Francisco State University, San Francisco, CA. 9 p. Miller, M. 1995. Amphibians survey, Birch Creek, July 1995. Unpublished report. University of California, Berkley. 3 pp.
Miller, M. 1995. Amphibians survey, Birch Creek, July 1995. Unpublished report. University of California, Berkley. 3 pp. Mittleman, M.B. 1948, American Caudata II. Geographic variation in Ambystoma macrodactylum. Herpetologica 4: 81-95.
Mittleman, M.B. 1948, American Caudata II. Geographic variation in Ambystoma macrodactylum. Herpetologica 4: 81-95. Monello, R.J. and R.G. Wright. 2001. Predation by goldfish (Carassius auratus) on eggs and larvae of the eastern long-toed salamander (Ambystoma macrodactylum columbianum). Journal of Herpetology 35(2): 350-353.
Monello, R.J. and R.G. Wright. 2001. Predation by goldfish (Carassius auratus) on eggs and larvae of the eastern long-toed salamander (Ambystoma macrodactylum columbianum). Journal of Herpetology 35(2): 350-353. Naughton, G.P., C.B. Henderson, K.R. Foresman, and R.L. McGraw, II. 2000. Long-toed salamanders in harvested and intact Douglas-fir forests of western Montana. Ecological Applications 10(6): 1681-1689.
Naughton, G.P., C.B. Henderson, K.R. Foresman, and R.L. McGraw, II. 2000. Long-toed salamanders in harvested and intact Douglas-fir forests of western Montana. Ecological Applications 10(6): 1681-1689. Nebeker, A.V., G.S. Schuytema, W.L. Griffis, and A. Cataldo. 1998. Impact of guthion on survival and growth of the frog Psuedacris regilla and the salamanders Ambystoma gracile and Ambystoma maculatum. Archives of Environmental Contamination and Toxicology 35(1):48-51.
Nebeker, A.V., G.S. Schuytema, W.L. Griffis, and A. Cataldo. 1998. Impact of guthion on survival and growth of the frog Psuedacris regilla and the salamanders Ambystoma gracile and Ambystoma maculatum. Archives of Environmental Contamination and Toxicology 35(1):48-51. Nelson, S.J., G.L. Powell, and A.P. Russell. 1995. Population survey of the long-toed salamander (Ambystoma macrodactylum) in southwestern Alberta. Alberta Environmental Protection, Fish and Wildlife Service, Edmonton, AB. 161pp.
Nelson, S.J., G.L. Powell, and A.P. Russell. 1995. Population survey of the long-toed salamander (Ambystoma macrodactylum) in southwestern Alberta. Alberta Environmental Protection, Fish and Wildlife Service, Edmonton, AB. 161pp. Northrop, Devine & Tarbell, Inc. 1994. Cabinet Gorge and Noxon Rapids hydroelectric developments: 1993 wildlife study. Unpublished report to the Washington Water Power Company, Spokane. Vancouver, Washington and Portland, Maine. 144 pp. plus appendices.
Northrop, Devine & Tarbell, Inc. 1994. Cabinet Gorge and Noxon Rapids hydroelectric developments: 1993 wildlife study. Unpublished report to the Washington Water Power Company, Spokane. Vancouver, Washington and Portland, Maine. 144 pp. plus appendices. Oseen, K.L., G.L. Powell, and A.P. Russell. 1995b. The distribution of the long-toed salamander (Ambystoma macrodactylum) in northwestern Alberta. Unpublished report submitted to Alberta Fish and Wildlife, Environmental Protection, Edmonton, Alberta. p
Oseen, K.L., G.L. Powell, and A.P. Russell. 1995b. The distribution of the long-toed salamander (Ambystoma macrodactylum) in northwestern Alberta. Unpublished report submitted to Alberta Fish and Wildlife, Environmental Protection, Edmonton, Alberta. p Palen, W.J., C.E. Williamson, A.A. Clauser, and D.E. Schindler. 2005. Impact of UV-B exposure on amphibian embryos: linking species physiology and oviposition behaviour. Proceedings of the Royal Society Biological Sciences Series B 272(1569):1227-1234.
Palen, W.J., C.E. Williamson, A.A. Clauser, and D.E. Schindler. 2005. Impact of UV-B exposure on amphibian embryos: linking species physiology and oviposition behaviour. Proceedings of the Royal Society Biological Sciences Series B 272(1569):1227-1234. Pearman, P.B. 2002. Interactions between Ambystoma salamander larvae: evidence for competitive asymmetry. Herpetologica 58(2):156-165.
Pearman, P.B. 2002. Interactions between Ambystoma salamander larvae: evidence for competitive asymmetry. Herpetologica 58(2):156-165. Pearson, K.J. 2004. Long-toed salamander (Ambystoma macrodactylum) Alberta Species at Risk Report 90:136-147.
Pearson, K.J. 2004. Long-toed salamander (Ambystoma macrodactylum) Alberta Species at Risk Report 90:136-147. Peters, W. 1882. Legte drei neue Batrachier (Amblystoma krausei, Nyctibatrachus sinensis, Bufo buchneri) vor. Sitzungs - bericht der Gesellschaft Naturforschender Freunde zu Berlin 10: 145-148.
Peters, W. 1882. Legte drei neue Batrachier (Amblystoma krausei, Nyctibatrachus sinensis, Bufo buchneri) vor. Sitzungs - bericht der Gesellschaft Naturforschender Freunde zu Berlin 10: 145-148. Powell, G.L., A.P. Russell, J.D. James, S.J. Nelson, and S.M. Watson. 1997. Population biology of the long-toed salamander (Ambystoma macrodactylum) in the front range of Alberta. In: D.M. Green (Ed.). Amphibians in decline: Canadian studies of a glob
Powell, G.L., A.P. Russell, J.D. James, S.J. Nelson, and S.M. Watson. 1997. Population biology of the long-toed salamander (Ambystoma macrodactylum) in the front range of Alberta. In: D.M. Green (Ed.). Amphibians in decline: Canadian studies of a glob Powell, G.L., A.P. Russell, S.J. Nelson, I.M. Hamilton, and K.L. Graham 1997. The status of the Long-toed salamander (Ambystoma macrodactylum) in Alberta. Alberta Environmental Protection, Fish and Wildlife Division, Edmonton, AB.
Powell, G.L., A.P. Russell, S.J. Nelson, I.M. Hamilton, and K.L. Graham 1997. The status of the Long-toed salamander (Ambystoma macrodactylum) in Alberta. Alberta Environmental Protection, Fish and Wildlife Division, Edmonton, AB. Powell, G.L., S.J. Nelson, and A.P. Russell. 1993. The Bow Valley long-toed salamander population study: a preliminary report on the 1992 field season. Alberta Environmental Protection, Fish and Wildlife Service, Edmonton, AB. 124 pp.
Powell, G.L., S.J. Nelson, and A.P. Russell. 1993. The Bow Valley long-toed salamander population study: a preliminary report on the 1992 field season. Alberta Environmental Protection, Fish and Wildlife Service, Edmonton, AB. 124 pp. Reed, R.J. 1978. Population study of the Santa-Cruz long-toed salamander (Ambystoma marcrodactylum croceum) at Valencia Lagoon 1977-1978 with notes on habitat and occurrence in Santa Cruz and Monterey countites. California Department of Fish and Game R
Reed, R.J. 1978. Population study of the Santa-Cruz long-toed salamander (Ambystoma marcrodactylum croceum) at Valencia Lagoon 1977-1978 with notes on habitat and occurrence in Santa Cruz and Monterey countites. California Department of Fish and Game R Reed, R.J. 1980. The population dynamics of Ambystoma macrodactylum croceum as a response to climatological variables. American Zoologist 20(4): 879.
Reed, R.J. 1980. The population dynamics of Ambystoma macrodactylum croceum as a response to climatological variables. American Zoologist 20(4): 879. Reichel, J. and D. Flath. 1995. Identification of Montana's amphibians and reptiles. Montana Outdoors 26(3):15-34.
Reichel, J. and D. Flath. 1995. Identification of Montana's amphibians and reptiles. Montana Outdoors 26(3):15-34. Reichel, J.D. 1995a. Preliminary amphibian and reptile survey of the Lewis & Clark National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 92 p.
Reichel, J.D. 1995a. Preliminary amphibian and reptile survey of the Lewis & Clark National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 92 p. Reichel, J.D. 1996. Preliminary amphibian and reptile survey of the Helena National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 87 pp.
Reichel, J.D. 1996. Preliminary amphibian and reptile survey of the Helena National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 87 pp. Reichel, J.D. 1997a. Amphibian, reptile and northern bog lemming survey on the Rocky Mountain Front: 1996. Montana Natural Heritage Program, Helena, MT. 81 p.
Reichel, J.D. 1997a. Amphibian, reptile and northern bog lemming survey on the Rocky Mountain Front: 1996. Montana Natural Heritage Program, Helena, MT. 81 p. Reichel, J.D. and S.G. Beckstrom. 1993. Northern bog lemming survey: 1992. Unpublished report. Montana Natural Heritage Program, Helena, MT. 64 p.
Reichel, J.D. and S.G. Beckstrom. 1993. Northern bog lemming survey: 1992. Unpublished report. Montana Natural Heritage Program, Helena, MT. 64 p. Rodgers, T. L. and W. L. Jellison. 1942. A collection of amphibians and reptiles from western Montana. Copeia (1):10-13.
Rodgers, T. L. and W. L. Jellison. 1942. A collection of amphibians and reptiles from western Montana. Copeia (1):10-13. Roedel, M.D. and P. Hendricks. 1998a. Amphibian and reptile survey on the Bureau of Land Management Lewistown District: 1995-1998. Montana Natural Heritage Program, Helena, MT. 53 p.
Roedel, M.D. and P. Hendricks. 1998a. Amphibian and reptile survey on the Bureau of Land Management Lewistown District: 1995-1998. Montana Natural Heritage Program, Helena, MT. 53 p. Roedel, M.D. and P. Hendricks. 1998b. Amphibian and reptile inventory on the Headwaters and Dillon Resource Areas in conjunction with Red Rocks Lakes National Wildlife Refuge: 1996-1998. Montana Natural Heritage Program, Helena, MT. 46 p.
Roedel, M.D. and P. Hendricks. 1998b. Amphibian and reptile inventory on the Headwaters and Dillon Resource Areas in conjunction with Red Rocks Lakes National Wildlife Refuge: 1996-1998. Montana Natural Heritage Program, Helena, MT. 46 p. Russell, A. P. and A. M. Bauer. 1993. The amphibians and reptiles of Alberta. University of Calgary Press. Calgary, Alberta. 264 p.
Russell, A. P. and A. M. Bauer. 1993. The amphibians and reptiles of Alberta. University of Calgary Press. Calgary, Alberta. 264 p. Russell, R.W. and J.D. Anderson. 1956. A disjunct population of the long-toed salamander from the coast of California. Herpetologica 12: 137-140.
Russell, R.W. and J.D. Anderson. 1956. A disjunct population of the long-toed salamander from the coast of California. Herpetologica 12: 137-140. Ruth, S.B. 1988. The life history and current status of the Santa Cruz long-toed salamander (Ambystoma macrodactylum croceum). Pages 89-110 in H.F. Lisle, P.R. Brown, B.Kaufman, and B.M. McGurty, editors. Proceedings of the Conference on California He
Ruth, S.B. 1988. The life history and current status of the Santa Cruz long-toed salamander (Ambystoma macrodactylum croceum). Pages 89-110 in H.F. Lisle, P.R. Brown, B.Kaufman, and B.M. McGurty, editors. Proceedings of the Conference on California He Ruth, S.B. and K. Tollestrup. 1973. Aspects of the life history and current status of the Santa Cruz long-toed salamander (Ambystoma macrodactylum croceum) at Valencia Lagoon, Santa Cruz County, California. Report for California Division of Highways. 5
Ruth, S.B. and K. Tollestrup. 1973. Aspects of the life history and current status of the Santa Cruz long-toed salamander (Ambystoma macrodactylum croceum) at Valencia Lagoon, Santa Cruz County, California. Report for California Division of Highways. 5 Schmidt, K.P. 1953. A checklist of North American Amphibians and Reptiles. Sixth edition. American Society of Icthyologists and Herpetologists. 280 p.
Schmidt, K.P. 1953. A checklist of North American Amphibians and Reptiles. Sixth edition. American Society of Icthyologists and Herpetologists. 280 p. Sheppard R.F. 1977. The ecology and home range movements of Ambystoma macrodactylum krausei (Amphibia : Urodela). M.S. Thesis, University of Calgary. 138pp.
Sheppard R.F. 1977. The ecology and home range movements of Ambystoma macrodactylum krausei (Amphibia : Urodela). M.S. Thesis, University of Calgary. 138pp. Sherratt, J. 2003. The effects of hydroperiod on the morphology of the long-toed salamanser Ambystoma macrodatylum. Undergraduate Honors Thesis. Carroll College, Helena, MT. 26 p.
Sherratt, J. 2003. The effects of hydroperiod on the morphology of the long-toed salamanser Ambystoma macrodatylum. Undergraduate Honors Thesis. Carroll College, Helena, MT. 26 p. Shoop, C.R. 1968. Migratory orientation of Ambystoma maculatum: movements near breeding ponds and displacements of migrating individuals. Biology Bulletin 135: 230-238.
Shoop, C.R. 1968. Migratory orientation of Ambystoma maculatum: movements near breeding ponds and displacements of migrating individuals. Biology Bulletin 135: 230-238. Spohr, S.M. 2005. Ambystoma maculatum (Spotted Salamander). Predation. Herpetological Review 36:294-295.
Spohr, S.M. 2005. Ambystoma maculatum (Spotted Salamander). Predation. Herpetological Review 36:294-295. Stebbins, R. C. 2003. A field guide to western reptiles and amphibians. 3rd Edition. Houghton Mifflin Company, Boston and New York. 533 p.
Stebbins, R. C. 2003. A field guide to western reptiles and amphibians. 3rd Edition. Houghton Mifflin Company, Boston and New York. 533 p. Talent, L.G. and C.L. Talent. 1980. A population of the endangered Santa Cruz long-toed salamander, Ambystoma macrodactylum croceum, from Monterey County, California. California Fish and Game 66(3):184-186.
Talent, L.G. and C.L. Talent. 1980. A population of the endangered Santa Cruz long-toed salamander, Ambystoma macrodactylum croceum, from Monterey County, California. California Fish and Game 66(3):184-186. Test, F.C. 1893. Annotated list of reptiles and batrachians collected. In B.W. Evermann. A reconnaisance of the streams and lakes of western Montana and northwestern Wyoming. Bulletin of United States Fish Commission 11(1891): 57-59.
Test, F.C. 1893. Annotated list of reptiles and batrachians collected. In B.W. Evermann. A reconnaisance of the streams and lakes of western Montana and northwestern Wyoming. Bulletin of United States Fish Commission 11(1891): 57-59. Thompson, M.D. 2000. Long-toed phylogeography a 'neo-perspective' on declines. The Boreal Dip Net 5(1): 14-16.
Thompson, M.D. 2000. Long-toed phylogeography a 'neo-perspective' on declines. The Boreal Dip Net 5(1): 14-16. Thompson, M.D. 2001. An unusually adept ambystomatid, the long toed salamander, coping at northern extremes. The Boreal Dip Net. 5(2):8-10.
Thompson, M.D. 2001. An unusually adept ambystomatid, the long toed salamander, coping at northern extremes. The Boreal Dip Net. 5(2):8-10. Thompson, M.D. and A.P. Russell. 2000. Phylogeography of Ambystoma macrodactylum: post glacial range expansion and resultant genetic diversity. Field Summary Report No. 1. University of Calgary. Calgary, Canada. 39 p.
Thompson, M.D. and A.P. Russell. 2000. Phylogeography of Ambystoma macrodactylum: post glacial range expansion and resultant genetic diversity. Field Summary Report No. 1. University of Calgary. Calgary, Canada. 39 p. Thompson, M.D. and A.P. Russell. 2001. Phylogeography of Ambystoma macrodactylum: post glacial range expansion and resultant genetic diversity. Field Summary Report No. 2. University of Calgary, Calgary, Canada. 65 p.
Thompson, M.D. and A.P. Russell. 2001. Phylogeography of Ambystoma macrodactylum: post glacial range expansion and resultant genetic diversity. Field Summary Report No. 2. University of Calgary, Calgary, Canada. 65 p. Thompson, Richard W., Western Resource Dev. Corp., Boulder, CO., 1996, Wildlife baseline report for the Montana [Montanore] Project, Lincoln and Sanders counties, Montana. In Application for a Hard Rock Operating Permit and Proposed Plan of Operation, Montanore Project, Lincoln and Sanders Counties, Montana. Vol. 5. Stroiazzo, John. Noranda Minerals Corp., Libby, MT. Revised September 1996.
Thompson, Richard W., Western Resource Dev. Corp., Boulder, CO., 1996, Wildlife baseline report for the Montana [Montanore] Project, Lincoln and Sanders counties, Montana. In Application for a Hard Rock Operating Permit and Proposed Plan of Operation, Montanore Project, Lincoln and Sanders Counties, Montana. Vol. 5. Stroiazzo, John. Noranda Minerals Corp., Libby, MT. Revised September 1996. Thoms, C., and C.C. Corkran. 1994. Identifying Oregon amphibians in the field: distinctions between long-toed and northwestern salamanders. Northwest Science 68(2): 154.
Thoms, C., and C.C. Corkran. 1994. Identifying Oregon amphibians in the field: distinctions between long-toed and northwestern salamanders. Northwest Science 68(2): 154. Tihen, J.A. 1969. Ambystoma. Catalogue of American Amphibians and Reptiles 75.1-75.4.
Tihen, J.A. 1969. Ambystoma. Catalogue of American Amphibians and Reptiles 75.1-75.4. Timken, R. No Date. Amphibians and reptiles of the Beaverhead National Forest. Western Montana College, Dillon, MT. 16 p.
Timken, R. No Date. Amphibians and reptiles of the Beaverhead National Forest. Western Montana College, Dillon, MT. 16 p. Turtle, S.L. 2000. Embryonic survivorship of the spotted salamander (Ambystoma maculatum) in roadside and woodland vernal pools in southeastern New Hampshire. Journal of Herpetology 34(1): 60-67.
Turtle, S.L. 2000. Embryonic survivorship of the spotted salamander (Ambystoma maculatum) in roadside and woodland vernal pools in southeastern New Hampshire. Journal of Herpetology 34(1): 60-67. Tyler, T.J. 1996. Interactions between stocked trout larval salamanders (Ambystoma macrodactylum) in high-elevation lakes. Masters Thesis, Oregon State University. 55p.
Tyler, T.J. 1996. Interactions between stocked trout larval salamanders (Ambystoma macrodactylum) in high-elevation lakes. Masters Thesis, Oregon State University. 55p. Vasconcelos, D. and A.J.K. Calhoun. 2004. Movement patterns of adult and juvenile Rana sylvatica (LeConte) and Ambystoma maculatum (Shaw) in three restored seasonal pools in Maine. Journal of Herpetology 38(4):551-561.
Vasconcelos, D. and A.J.K. Calhoun. 2004. Movement patterns of adult and juvenile Rana sylvatica (LeConte) and Ambystoma maculatum (Shaw) in three restored seasonal pools in Maine. Journal of Herpetology 38(4):551-561. Verrell, P. and J. Pelton. 1996. The sexual strategy of the central long-toed salamander, Ambystoma macrodactylum columbianum, in south-eastern Washington. Journal of the Zoological Society of London 240: 37-50.
Verrell, P. and J. Pelton. 1996. The sexual strategy of the central long-toed salamander, Ambystoma macrodactylum columbianum, in south-eastern Washington. Journal of the Zoological Society of London 240: 37-50. Verrell, P. and K. Davis. 2003. Do non-breeding, adult long-toed salamanders respond to conspecifics as friends or as foes? Herpetologica 59(1): 1-7.
Verrell, P. and K. Davis. 2003. Do non-breeding, adult long-toed salamanders respond to conspecifics as friends or as foes? Herpetologica 59(1): 1-7. Verrell, P., N. Strand, and E.Hanson. Sexual dimorphism of mate location in the long-toed salamander Ambystoma macrodactylum columbianum. Ethology 107(8): 677-684.
Verrell, P., N. Strand, and E.Hanson. Sexual dimorphism of mate location in the long-toed salamander Ambystoma macrodactylum columbianum. Ethology 107(8): 677-684. Verrell, P.A. 2004. The male reproductive cycle of the North American salamander Ambystoma macrodactylum columbianum. Amphibia-Reptilia 25(3):349-356.
Verrell, P.A. 2004. The male reproductive cycle of the North American salamander Ambystoma macrodactylum columbianum. Amphibia-Reptilia 25(3):349-356. Walker, R.S. 1984. Distribution of the northern long-toed salamander (Ambystoma macrodactylum) east of the continental divide in Montana. Honors thesis, Carroll College. Helena, MT. 37 pp.
Walker, R.S. 1984. Distribution of the northern long-toed salamander (Ambystoma macrodactylum) east of the continental divide in Montana. Honors thesis, Carroll College. Helena, MT. 37 pp. Walls, S.C. and A.R. Blaustein. 1994. Does kinship influence density dependence in a larval salamander? Oikos 71(3):459-468.
Walls, S.C. and A.R. Blaustein. 1994. Does kinship influence density dependence in a larval salamander? Oikos 71(3):459-468. Walls, S.C., S.S. Belanger, and A.R. Blaustein. 1993. Morphological variation in a larval salamander: dietary induction of plasticity in head shape. Oecologica 96:162-168.
Walls, S.C., S.S. Belanger, and A.R. Blaustein. 1993. Morphological variation in a larval salamander: dietary induction of plasticity in head shape. Oecologica 96:162-168. Walsh, R. 1998. An extension of the known range of the long-toed salamander, Ambystoma macrodactylum, in Alberta. Canadian Field Naturalist 112: 331-333.
Walsh, R. 1998. An extension of the known range of the long-toed salamander, Ambystoma macrodactylum, in Alberta. Canadian Field Naturalist 112: 331-333. Watson S. 1997. Food level effects on metamorphic timing in the long-toed salamander, Ambystoma macrodactylum krausei. Masters Thesis, Univesisty of Calgary. pp. 206.
Watson S. 1997. Food level effects on metamorphic timing in the long-toed salamander, Ambystoma macrodactylum krausei. Masters Thesis, Univesisty of Calgary. pp. 206. Weisel, G.F. 1952. Animal names, anatomical terms, and some ethnozoology of the Flathead Indians. Journal of the Washington Academy of Sciences 42(11): 345-355.
Weisel, G.F. 1952. Animal names, anatomical terms, and some ethnozoology of the Flathead Indians. Journal of the Washington Academy of Sciences 42(11): 345-355. Werner, J.K. and J.D. Reichel. 1994. Amphibian and reptile survey of the Kootenai National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 104 p.
Werner, J.K. and J.D. Reichel. 1994. Amphibian and reptile survey of the Kootenai National Forest: 1994. Montana Natural Heritage Program. Helena, MT. 104 p. Werner, J.K. and J.D. Reichel. 1996. Amphibian and reptile monitoring/survey of the Kootenai National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 115 pp.
Werner, J.K. and J.D. Reichel. 1996. Amphibian and reptile monitoring/survey of the Kootenai National Forest: 1995. Montana Natural Heritage Program. Helena, MT. 115 pp. Werner, J.K. and T. Plummer. 1995a. Amphibian and reptile survey of the Flathead Indian Reservation 1993-1994. Salish Kootenai College, Pablo, MT. 55 pp.
Werner, J.K. and T. Plummer. 1995a. Amphibian and reptile survey of the Flathead Indian Reservation 1993-1994. Salish Kootenai College, Pablo, MT. 55 pp. Werner, J.K. and T. Plummer. 1995b. Amphibian monitoring program on the Flathead Indian Reservation 1995. Salish Kootenai College, Pablo, MT. 46 p.
Werner, J.K. and T. Plummer. 1995b. Amphibian monitoring program on the Flathead Indian Reservation 1995. Salish Kootenai College, Pablo, MT. 46 p. Werner, J.K., T. Plummer, and J. Weaselhead. 1998b. The status of amphibians on the Flathead Reservation, Montana. Intermountain Journal of Sciences 4(3-4): 88.
Werner, J.K., T. Plummer, and J. Weaselhead. 1998b. The status of amphibians on the Flathead Reservation, Montana. Intermountain Journal of Sciences 4(3-4): 88. Werner, J.K., T. Plummer, and J. Weaslehead. 1998a. Amphibians and reptiles of the Flathead Indian Reservation. Intermountain Journal of Sciences 4(1-2): 33-49.
Werner, J.K., T. Plummer, and J. Weaslehead. 1998a. Amphibians and reptiles of the Flathead Indian Reservation. Intermountain Journal of Sciences 4(1-2): 33-49. Wildy, E.L., and A.R. Blaustein. 2001. Learned recognition of intraspecific predators in larval long-toed salamanders Ambystoma macrodactylum. Ethology 107(6): 479-494.
Wildy, E.L., and A.R. Blaustein. 2001. Learned recognition of intraspecific predators in larval long-toed salamanders Ambystoma macrodactylum. Ethology 107(6): 479-494. Wildy, E.L., D.P. Chivers, and A.R. Blaustein. 1999. Shifts in life history trairs as a response to cannibalism in larval long-toed salamanders (Ambystoma macrodactylum). Journal of Chemical Ecology 25: 2337-2346.
Wildy, E.L., D.P. Chivers, and A.R. Blaustein. 1999. Shifts in life history trairs as a response to cannibalism in larval long-toed salamanders (Ambystoma macrodactylum). Journal of Chemical Ecology 25: 2337-2346. Wildy, E.L., D.P. Chivers, J.M. Kiesecker, and A.R. Blaustein. 1998. Cannibalism enhances growth in larval long-toed salamanders, (Ambystoma macrodactylum). Journal of Herpetology 32(2) 286-289.
Wildy, E.L., D.P. Chivers, J.M. Kiesecker, and A.R. Blaustein. 1998. Cannibalism enhances growth in larval long-toed salamanders, (Ambystoma macrodactylum). Journal of Herpetology 32(2) 286-289. Wildy, E.L., D.P. Chivers, J.M. Kiesecker, and A.R. Blaustein. 2001. The effects of food level and conspecific density on biting and cannibalism in larval long-toed salamanders, Ambystoma macrodactylum. Oecologial 128(2): 202-209.
Wildy, E.L., D.P. Chivers, J.M. Kiesecker, and A.R. Blaustein. 2001. The effects of food level and conspecific density on biting and cannibalism in larval long-toed salamanders, Ambystoma macrodactylum. Oecologial 128(2): 202-209. Williams, T.A., and C.D. Anthony. 1994. Technique to isolate salamander granular gland products with a comment on the evolution of adhesiveness. Copeia 1994(2): 540-541.
Williams, T.A., and C.D. Anthony. 1994. Technique to isolate salamander granular gland products with a comment on the evolution of adhesiveness. Copeia 1994(2): 540-541. Williams, T.A., and J.H. Larsen, Jr. 1986. New function for the granular skin glands of the eastern long-toed salamander, Ambystoma macrodactylum columbianum. Journal of Experimental Zoology 239(3): 329-334.
Williams, T.A., and J.H. Larsen, Jr. 1986. New function for the granular skin glands of the eastern long-toed salamander, Ambystoma macrodactylum columbianum. Journal of Experimental Zoology 239(3): 329-334. Wirsing, A.J., J.D. Roth, and D.L. Murray. 2005. Can prey use dietary cues to distinguish predators? A test involving three terrestrial amphibians. Herpetologica 61(2):104-110.
Wirsing, A.J., J.D. Roth, and D.L. Murray. 2005. Can prey use dietary cues to distinguish predators? A test involving three terrestrial amphibians. Herpetologica 61(2):104-110. Wright, A.N. and K.R. Zamudio. 2002. Color pattern asymmetry as a correlate of habitat disturbance in spotted salamanders (Ambystoma maculatum). Journal of Herpetology 36(1):129-133.
Wright, A.N. and K.R. Zamudio. 2002. Color pattern asymmetry as a correlate of habitat disturbance in spotted salamanders (Ambystoma maculatum). Journal of Herpetology 36(1):129-133. Zackheim, K. 1973. Exhibit H: Wildlife Study. In Ash Grove Cement Co. files.
Zackheim, K. 1973. Exhibit H: Wildlife Study. In Ash Grove Cement Co. files. Zalisko, E.J. 1987. Morphology, histochemistry, and seasonal changes in the vasa deferentia of Rhyacotriton olympicus olympicus (Dicamptodontidae) and Ambystoma macrodactylum columbianum (Ambystomatidae), (Amphibia, Urodela). Ph.D. Dissertation. Washi
Zalisko, E.J. 1987. Morphology, histochemistry, and seasonal changes in the vasa deferentia of Rhyacotriton olympicus olympicus (Dicamptodontidae) and Ambystoma macrodactylum columbianum (Ambystomatidae), (Amphibia, Urodela). Ph.D. Dissertation. Washi Zalisko, E.J. and J.H. Larsen Jr. 1990. Breeding and post-breeding structure of the vas deferens of the long-toed salamander Ambystoma macrodactylum. Journal of Morphology 203(3): 321-330.
Zalisko, E.J. and J.H. Larsen Jr. 1990. Breeding and post-breeding structure of the vas deferens of the long-toed salamander Ambystoma macrodactylum. Journal of Morphology 203(3): 321-330. Zalisko, E.J. and J.H. Larsen, Jr. 1989. Fate of unused sperm in post-breeding male Ambystoma macrodactylum columbianum. Journal of Herpetology 23(4): 463-464.
Zalisko, E.J. and J.H. Larsen, Jr. 1989. Fate of unused sperm in post-breeding male Ambystoma macrodactylum columbianum. Journal of Herpetology 23(4): 463-464. Zamudio, K.R. and W.K. Savage. 2003. Historical isolation, range expansion, and secondary contact of two highly divergent mitochondrial lineages in spotted salamanders (Ambystoma maculatum). Evolution 57:1631-1652.
Zamudio, K.R. and W.K. Savage. 2003. Historical isolation, range expansion, and secondary contact of two highly divergent mitochondrial lineages in spotted salamanders (Ambystoma maculatum). Evolution 57:1631-1652. Zisook, R., K. Almond, and B. Sharpe. 1996. Amphibian survey of the Birch Creek drainage, Beaverhead County. Wildland Studies Project. San Francisco State University, San Francisco, CA. 9 p.
Zisook, R., K. Almond, and B. Sharpe. 1996. Amphibian survey of the Birch Creek drainage, Beaverhead County. Wildland Studies Project. San Francisco State University, San Francisco, CA. 9 p.
- Web Search Engines for Articles on "Long-toed Salamander"
- Additional Sources of Information Related to "Amphibians"





